Table of Contents
Overview – Chemotherapy Drugs
Chemotherapy drugs are central to the treatment of cancer, aiming to eliminate malignant cells while preserving normal tissue. Historically, chemotherapy targeted rapidly dividing cells, but this non-specific approach led to substantial side effects. Modern strategies now include targeting tumour blood supply and utilising immune-modulating agents. This article outlines the pharmacology, mechanisms, uses, and adverse effects of cytotoxic and hormonal agents, as well as novel biological therapies in cancer care.
Principles of Chemotherapy
Traditional vs. Modern Focus
- Old Approach: Target rapid DNA replication and metabolic activity in dividing cells
- Limitations:
- Not all cancer cells divide rapidly
- Normal rapidly-dividing tissues are also affected (e.g. GI mucosa, bone marrow, skin, hair, gametes)
- Limitations:
- Newer Approaches:
- Anti-angiogenesis: Starve tumour of blood supply
- Combination therapy: Combine cytotoxic and targeted agents for better efficacy
Key Goal: Selective Toxicity
- Aim to kill cancer cells without harming normal tissues (still largely unmet)
- Strategies to improve selectivity:
- Drug screening and optimisation
- Target unique tumour antigens or environments
Mechanisms of Action
Cell Cycle Specificity
- S-phase specific: Inhibit DNA synthesis (e.g. antimetabolites)
- M-phase specific: Disrupt mitosis (e.g. plant alkaloids)
- Phase-nonspecific: Affect metabolism across all phases (e.g. alkylating agents)
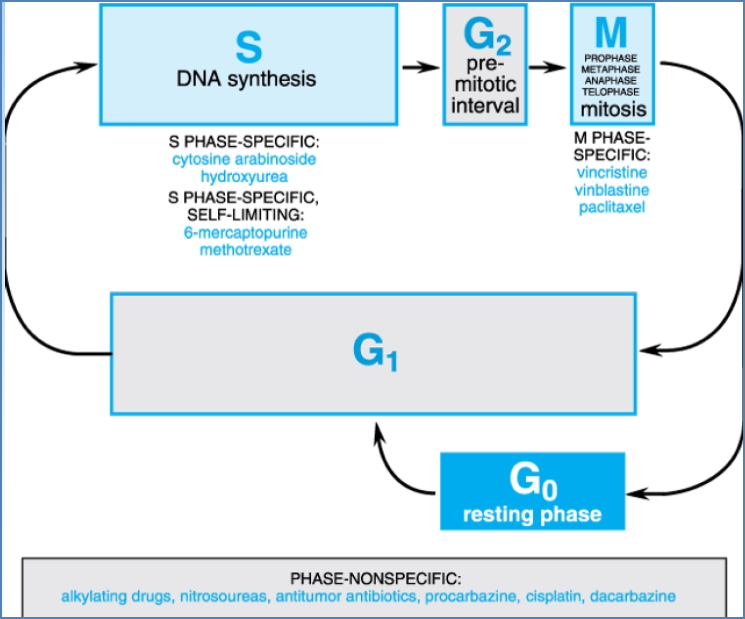

Cytotoxic Drug Classes
Alkylating Agents
- Mechanism: Form DNA cross-links → Disrupt replication/transcription → Apoptosis
- Drugs: Cyclophosphamide, Cisplatin, Nitrosoureas
- Indications: Lymphomas, leukaemias, breast, ovarian, testicular, bladder, endometrial cancers
- Side Effects: Myelosuppression, GI upset, infertility, rare secondary malignancies


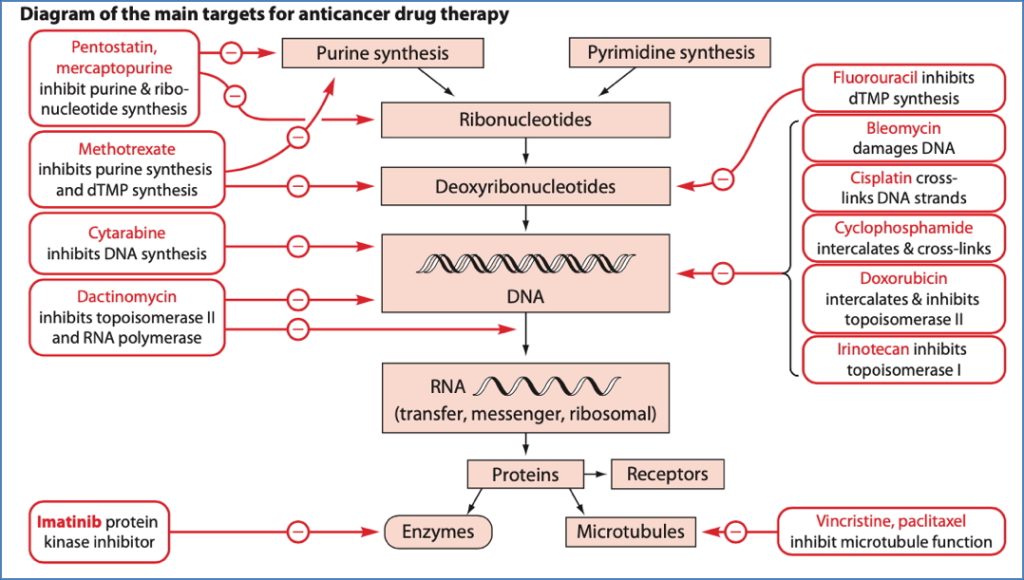
Antimetabolites
- Mechanism: Mimic normal substrates → Disrupt DNA/RNA synthesis
- Types:
- Folate antagonists (e.g. Methotrexate)
- Inhibits dihydrofolate reductase → Blocks purine/thymidylate synthesis
- Pyrimidine analogues (e.g. Fluorouracil)
- Inhibits thymidylate synthase → Fraudulent nucleotides
- Purine analogues (e.g. Mercaptopurine, Azathioprine)
- Interfere with adenine/guanine synthesis
- Folate antagonists (e.g. Methotrexate)
- Side Effects: Myelosuppression, hepatotoxicity (purine analogues), GI upset
Cytotoxic Antibiotics
- Mechanism: Intercalate DNA, inhibit topoisomerase, generate free radicals
- Drugs: Doxorubicin, Dactinomycin, Bleomycin
- Indications: Leukaemias, lymphomas, breast, soft tissue cancers
- Side Effects: Myelosuppression, cardiotoxicity (Bleomycin), alopecia, nausea


Plant Alkaloids
- Vinca alkaloids (e.g. Vincristine): Inhibit microtubule polymerisation
- Taxanes (e.g. Paclitaxel): Stabilise microtubules → Mitotic arrest
- Camptothecins (e.g. Irinotecan): Inhibit topoisomerase I
- Side Effects: Neurotoxicity (vinca), myelosuppression, GI upset

Hormonal Therapies
Hormone Agonists/Antagonists
- Glucocorticoids: Prednisone, dexamethasone (haematological cancers)
- Oestrogens: Diethylstilbestrol (prostate cancer, palliative)
- Progestogens: Megestrol (endometrial, renal cancers)
- Anti-oestrogens:
- SERMs (e.g. Tamoxifen) → Breast cancer
- SERDs (e.g. Fulvestrant) → Receptor downregulation
- Anti-androgens: Flutamide (prostate cancer)
Inhibitors of Hormone Synthesis
- GnRH agonists/antagonists: Suppress LH/FSH → Reduce sex hormones
- Aromatase inhibitors: Anastrozole, Letrozole (postmenopausal breast cancer)
- 5α-Reductase inhibitors: Finasteride (benign prostatic hypertrophy)
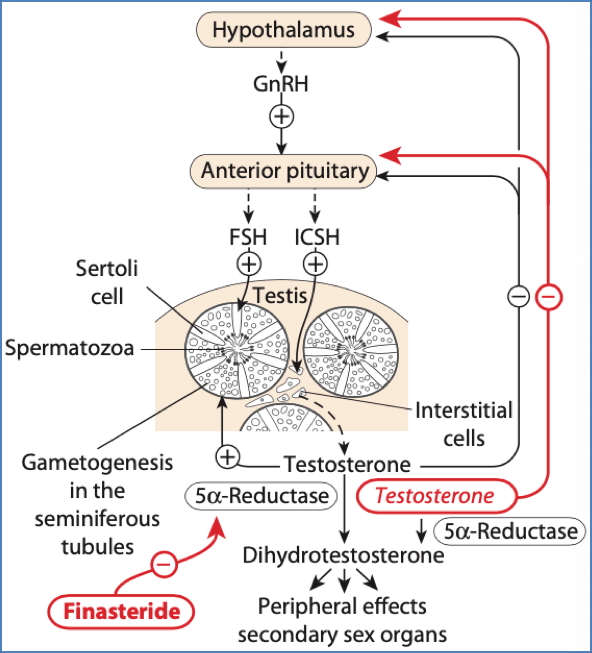



2. Smith, I.E., & Dowsett, M. (2003). Aromatase inhibitors in breast cancer. The New England journal of medicine, 348 24, 2431-42 .
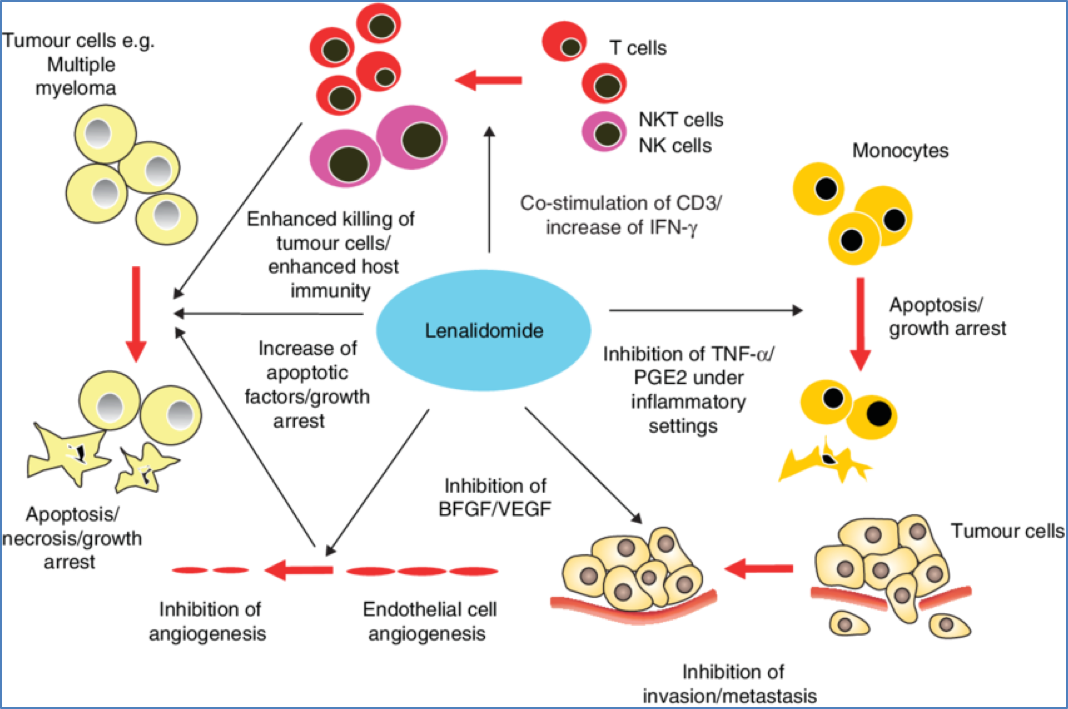
Thalidomide and Analogues
- Mechanisms:
- Inhibits angiogenesis
- Modulates immune function (e.g. ↑ NK cell activity)
- Promotes apoptosis
- Indications: Multiple myeloma
- Side Effects: Severe foetal malformation (strict contraindication in pregnancy)

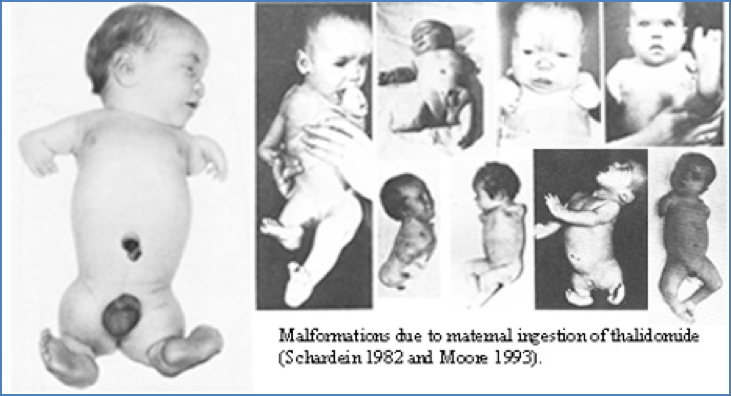

2. https://upload.wikimedia.org/wikipedia/commons/c/cd/Thalidomide_effects.jpg
Biological Therapies
Immunomodulators
- Interferons: Enhance NK/T-cell activity, inhibit proliferation
- Interleukin-2: Stimulate lymphocytes in vitro → Re-infuse to attack tumour
Monoclonal Antibodies
- Mechanism: Target tumour antigens or secreted growth factors
- Examples: Rituximab, Trastuzumab, Cetuximab
- Indications: Lymphomas, CLL, solid tumours
- Challenges: High specificity required; immunogenicity; complex dosing
General Side Effects of Chemotherapy
- Alopecia
- Nausea/vomiting
- Myelosuppression
- GI ulcers and mucositis
- Infertility
- Organ toxicity (heart, liver, kidneys, CNS)
Treatment Complications (Not Side Effects)
Drug Resistance
- Due to tumour mutations
- Management: Multi-drug therapy, adjusted timelines
Tumour Sanctuaries
- Sites where drugs poorly penetrate (e.g. CNS, encapsulated tumours)
- Management: Surgery, radiotherapy
Dose Exhaustion
- Reaching max tolerated dose limits further use
- Management: Combined modality treatment
Myelosuppression
- Suppressed myeloid lineages
- Management: Bone marrow stimulation via growth factors
Summary – Chemotherapy Drugs
Chemotherapy drugs remain a cornerstone in the management of cancer, targeting rapidly dividing tumour cells through diverse mechanisms such as DNA disruption, mitotic interference, and hormone modulation. Although traditional cytotoxic agents like alkylating agents and antimetabolites are effective, they also affect rapidly dividing healthy tissues, leading to notable side effects. Modern approaches, including monoclonal antibodies and biological response modifiers, aim for greater tumour selectivity. A strong understanding of these agents, their mechanisms, indications, and complications is essential for all medical students and junior doctors. For a broader context, see our Pharmacology & Toxicology Overview page.