Table of Contents
Overview
Genetics of cancer is a cornerstone topic in modern oncology, exploring how specific mutations in proto-oncogenes, tumour suppressor genes, and DNA repair pathways drive malignant transformation. At its core, cancer arises from clonal expansion of a single mutated cell and evolves through genetic instability and natural selection. While many mutations are sporadic, cancer can also be hereditary or virally induced, with progression often accelerated by environmental carcinogens. Understanding the molecular pathways behind uncontrolled cell division, apoptosis evasion, and metastasis is critical for both clinical practice and future therapeutic development.
Cancer Cells are Clonal
- All cancer cells in a tumour originate from a single progenitor cell that underwent neoplastic transformation.
- Stem cells may fail to produce differentiated progeny.
- Or, daughter cells may proliferate abnormally.
- Often involves a combination of both.


Selection of Traits in Cancer Cells
Cancer cells evolve via selection for advantageous traits that promote tumour survival and expansion:
- Increased proliferation rate
- Increased genomic instability (to promote further mutation)
- Tissue invasion and metastasis
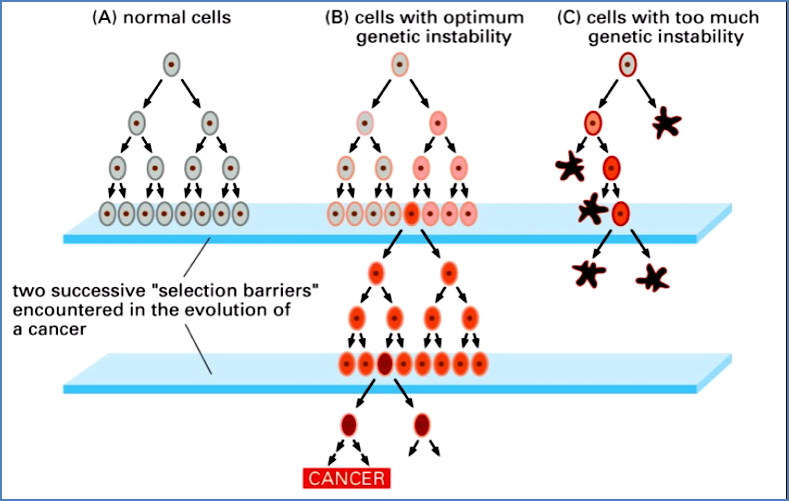

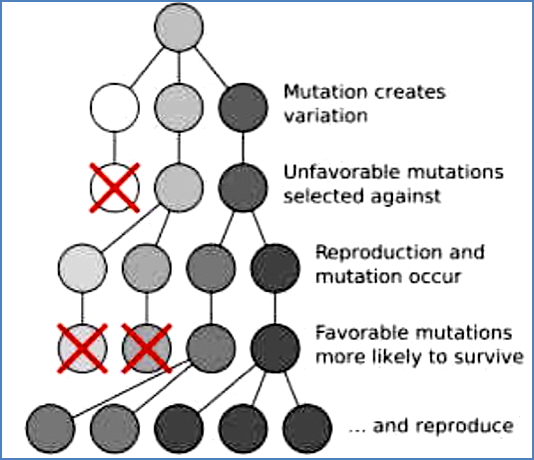

Note: Cells that are too unstable genetically are selected against—they can’t survive.
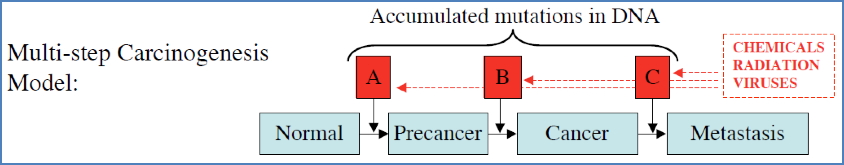
Cancer: Genetic Disease with Environmental Input
- Results from accumulated DNA mutations in several key genes (often ≥4).
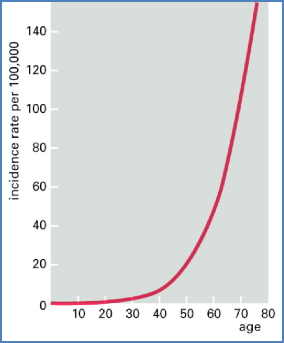
- Occurs over time → Cancer risk increases with age.
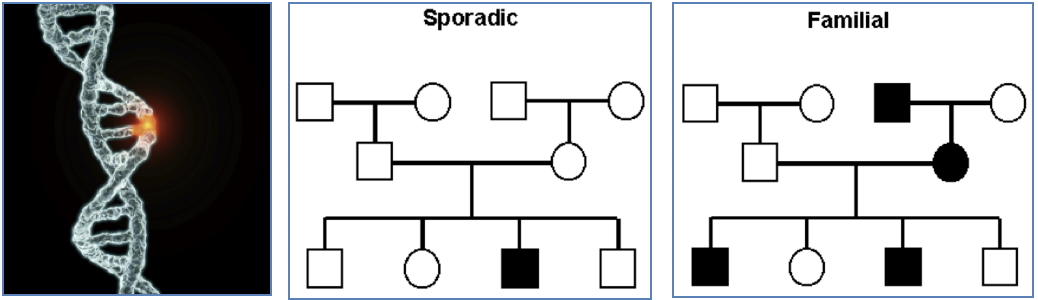
- Can be hereditary (germline mutation) or sporadic (somatic mutation).


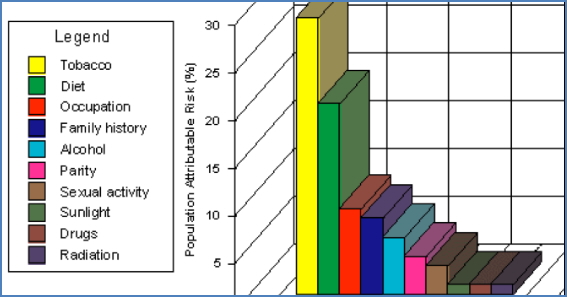
Environmental & Viral Factors
- Carcinogens: Tobacco, alcohol, radiation, chemicals
- Some act synergistically (e.g., tobacco + alcohol).
- Viruses:
- Viruses force host cells to divide by inactivating RB protein.
- Host p53 detects this abnormality and may induce apoptosis.
- Viruses counter by inactivating p53 as well.




Why Don’t We All Have Cancer?
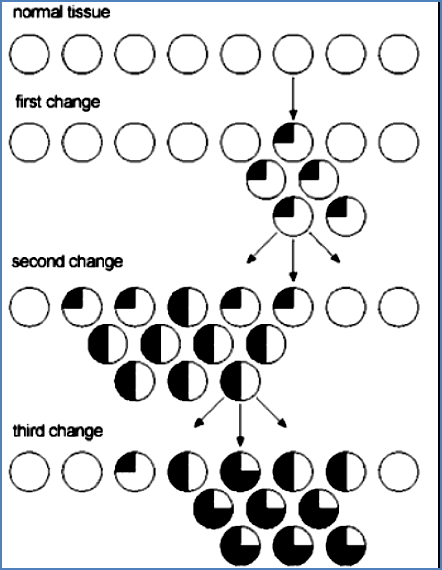
- Billions of DNA bases are copied constantly.
- But: Cancer requires several sequential mutations in specific genes in a single cell.
- Probability of this is low, but:
- Some mutations increase proliferation → more cells = more mutation targets
- Others increase instability → more mutations

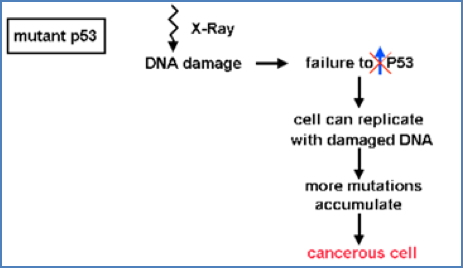
p53: The Guardian of the Genome
- Most frequently mutated gene in cancer (50–60% of tumours)
- Activated in response to DNA damage (via ATM/ATR pathways)
- Induces transcription of p21, which inhibits S-Cyclin-CDK → halts cell cycle
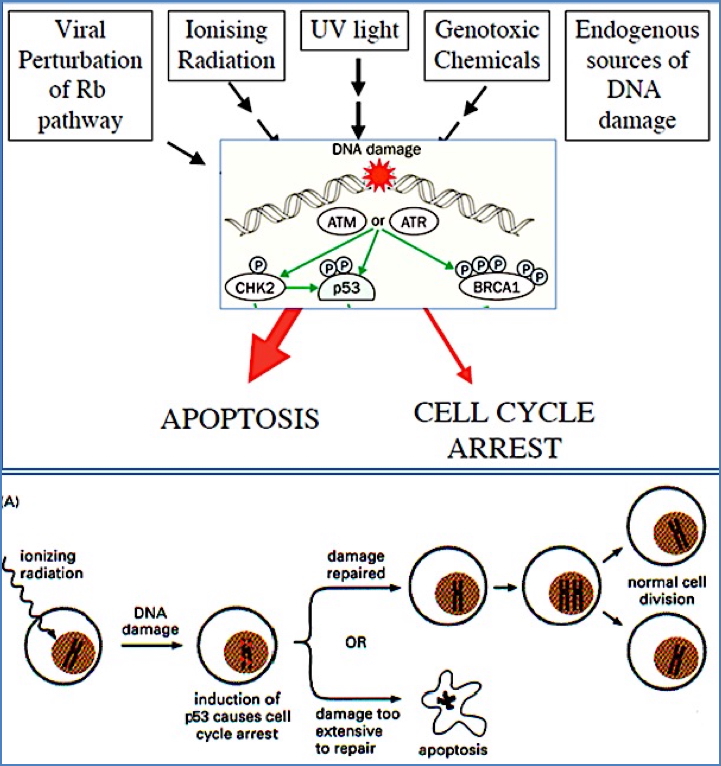



Loss of p53:
- Cells proceed through S phase with damaged DNA
- Leads to chromosomal instability and further mutations



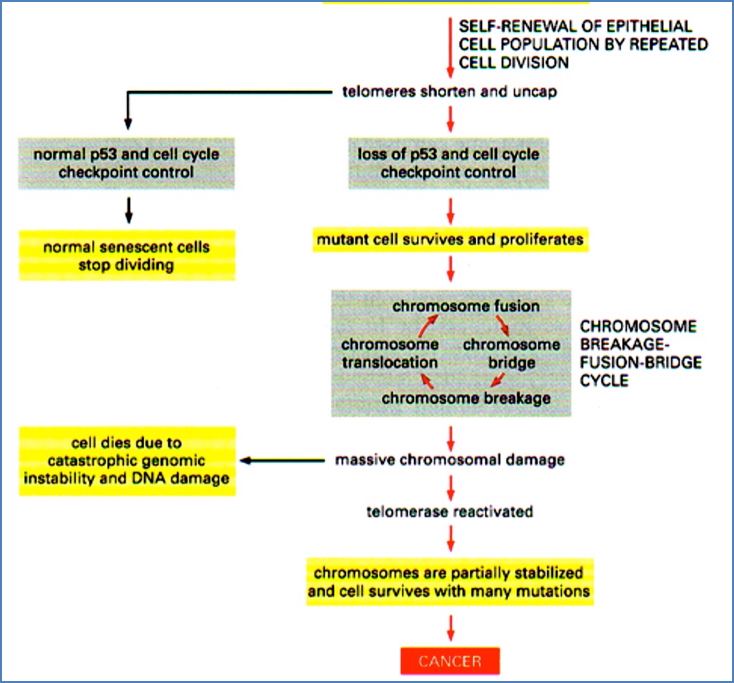
Chromosomal Instability: Breakage-Fusion-Bridge (BFB) Cycle
- A chromosome lacking a telomere undergoes replication
- Ends fuse, forming a bridge
- During mitosis, bridge breaks randomly
- Daughter cells inherit broken chromosomes → cycle repeats
→ Accelerated mutation rate



Cell-Cycle Checkpoints & Cancer
Key Cyclin-CDK Checkpoints:
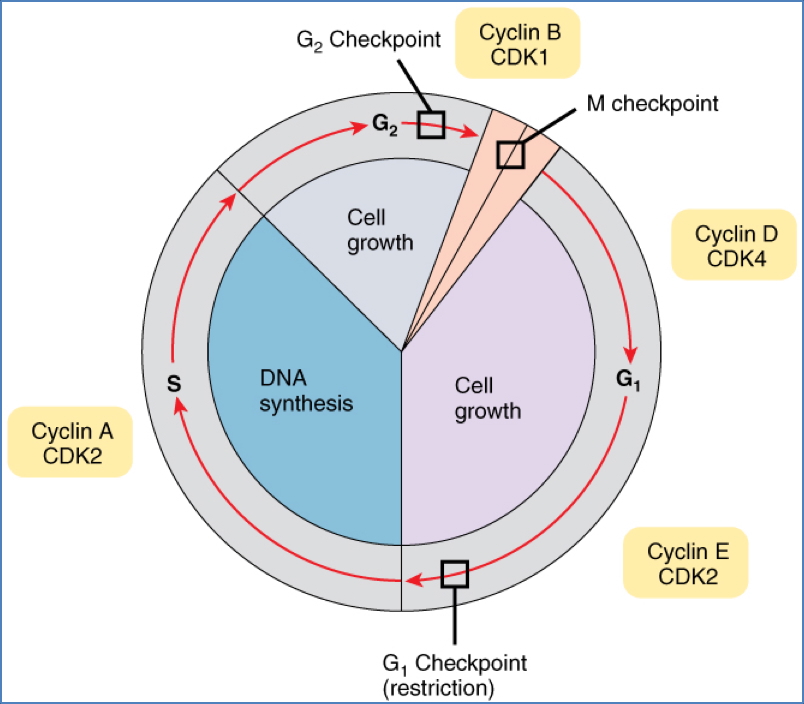

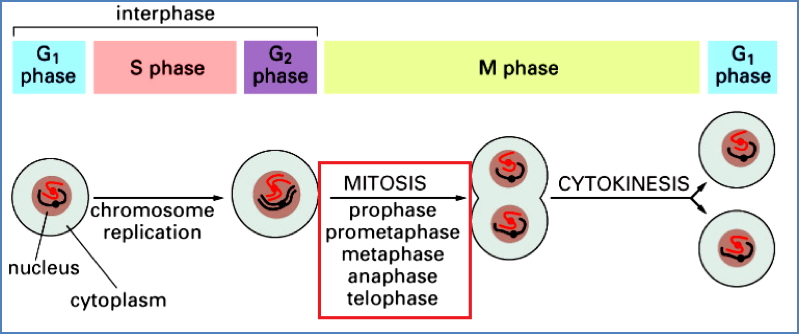

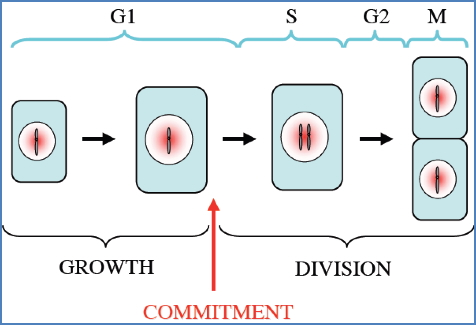

- G1/S Phase:
- Checks DNA integrity & environment
- Most commonly dysregulated in cancer
- p53 → p21 → inhibits S-Cyclin-CDK if damage is detected
- Mutation in p53 → cells replicate damaged DNA
- G2/M Phase:
- Checks DNA replication & environment
- Rb protein inhibits E2F (a transcription factor)
- If Rb is phosphorylated, E2F is released → triggers division
- Mutation in Rb → unchecked cell division


Many viruses inactivate both p53 and Rb, hijacking the cell cycle.
Categories of Genes Altered in Cancer
1. Gatekeepers (Proto-Oncogenes)
- Promote cell proliferation
- Mutation = Gain of function → becomes oncogene
- Dominant: Only one copy needs to be mutated
- Functions: Growth factors, receptors, transcription factors
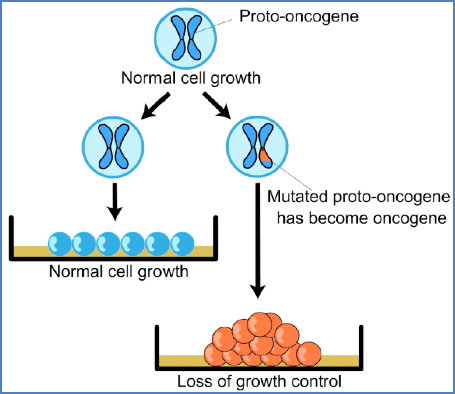



Mechanisms of Activation:
- Point mutations
- Gene amplification (e.g., double minute chromosomes, homogenously staining regions)
- Chromosomal rearrangements
- e.g., Philadelphia chromosome (BCR-ABL fusion in leukaemia)


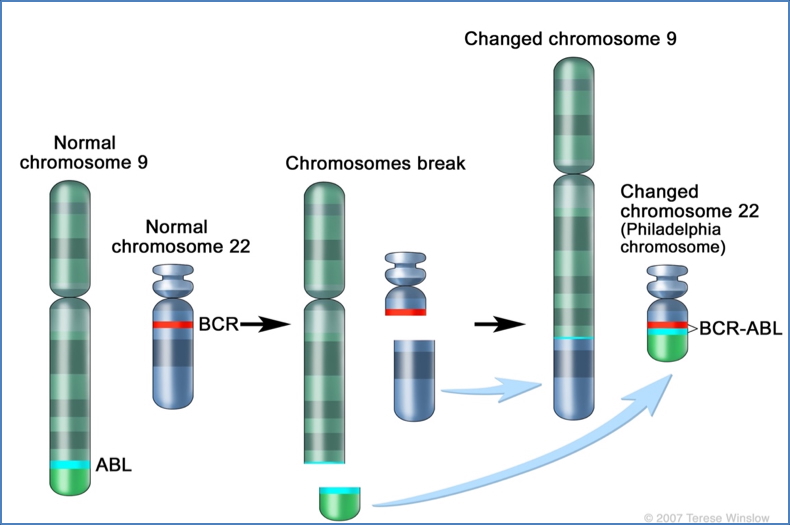

2. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/philadelphia-chromosome
2. Caretakers (Tumour Suppressor Genes)
- Maintain genomic stability (DNA repair, apoptosis, checkpoint control)
- Mutation = Loss of function → recessive (requires both alleles)
- Examples:
- p53, RB (cell-cycle regulation)
- BRCA1/2 (DNA repair)
- P16, ATM, APC
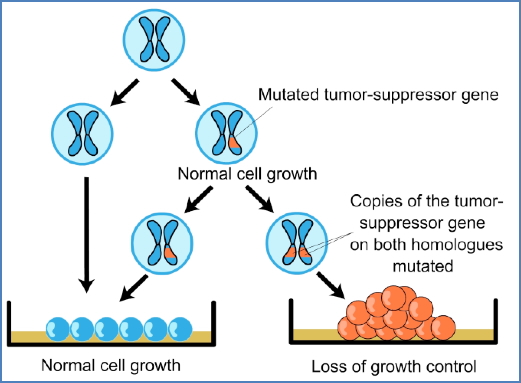



Knudson’s Two-Hit Hypothesis:
- Sporadic cancers: two somatic mutations
- Inherited cancers: one germline mutation + one somatic “hit”


Loss of Heterozygosity (LOH):
- Loss of the functional wild-type allele
- Occurs via:
- Chromosome deletion
- Mitotic recombination
- Gene conversion
- Epigenetic silencing


3. Landscapers
- Regulate cell adhesion, tissue architecture, and movement
- Mutation → promotes metastasis
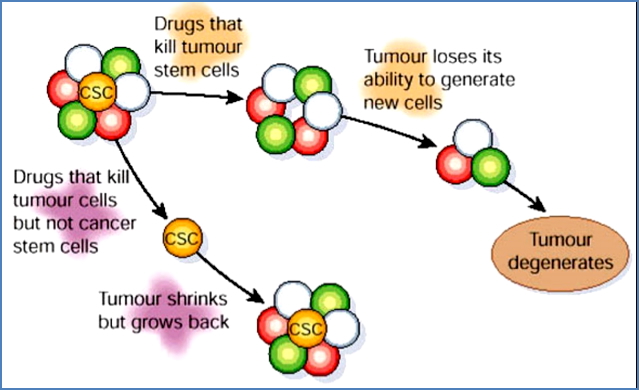
Cancer Stem Cells (CSCs)
- Rare cells in tumours with self-renewal capacity
- Drive tumour growth and recurrence
Classical vs CSC Theory:
| Classical Model | CSC Model |
|---|---|
| All tumour cells are equal | Only CSCs drive tumour growth |
| Tumour = hyper-proliferative | Tumour = stem cell disorder |
Therapeutic Implications:
- Most drugs target proliferating tumour cells
- CSCs may be quiescent → evade standard therapy
- New treatments must target CSCs to prevent relapse

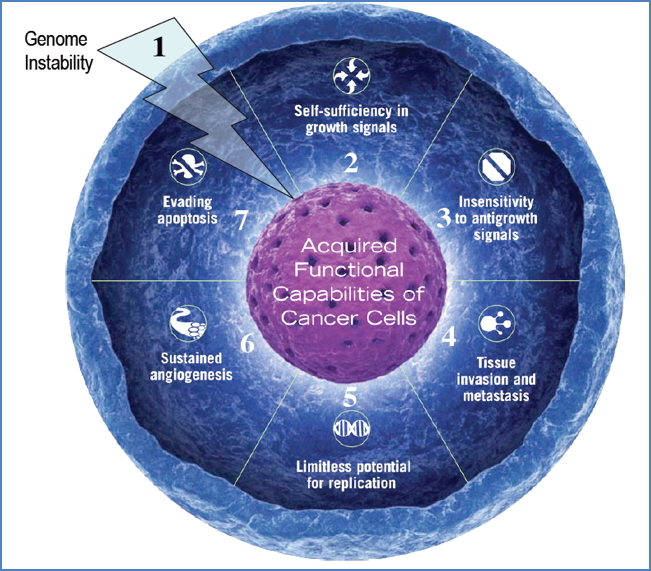
Summary of Key Genetic Hallmarks of Cancer
- Genome Instability
- Failure of DNA repair & checkpoint systems
- BFB cycle, chromosomal abnormalities
- Self-Sufficiency in Growth Signals
- Oncogene activation → constant proliferation
- Insensitivity to Anti-Growth Signals
- Tumour suppressor gene inactivation (e.g., RB)
- Tissue Invasion & Metastasis
- Loss of cell-cell adhesion genes
- Limitless Replicative Potential
- Reactivation of telomerase → cellular immortality
- Sustained Angiogenesis
- VEGF secretion → new blood vessel formation
- Evasion of Apoptosis
- Loss of pro-apoptotic genes; activation of anti-apoptotic pathways

Why Do People Die of Cancer?
- Cancer cells invade vital organs, impairing function
- Mortality stats:
- ~10% due to the primary tumour
- ~90% due to metastasis
- Metastases are responsible for most cancer-related deaths
- ~50% of surgically removed cancers recur
- Only ~10% of metastatic cancers are curable by chemotherapy/radiation
Summary – Genetics of Cancer
The genetics of cancer explains how somatic mutations in proto-oncogenes, tumour suppressors, and DNA repair pathways lead to malignant transformation. Cancer cells evolve through selection for traits like unchecked proliferation, evasion of apoptosis, and invasion, often via dysregulation of p53 and RB. The cancer stem cell theory provides additional insight into relapse and treatment resistance. For a broader context, see our Genetics & Cancer Overview page.