Table of Contents
Overview – B-Cell Development
B-cell development is the multistep process by which hematopoietic stem cells mature into antibody-secreting plasma cells. This occurs through defined stages in the bone marrow and peripheral lymphoid organs. The process involves immunoglobulin gene rearrangement, negative selection to prevent autoimmunity, migration to secondary lymphoid tissues, antigen-dependent activation, and finally, differentiation into effector cells. Understanding B-cell development is crucial for appreciating how adaptive humoral immunity is generated and regulated.
Development in Bone Marrow
Step 1 – Ig Gene Rearrangement
- Initiated by signals from bone marrow stromal cells
- Heavy chain rearrangement:
- Random recombination of one gene each from the V (variable), D (diversity), and J (joining) loci
- Light chain rearrangement:
- Involves only V and J gene loci
- Key enzymes involved:
- RAG-1 and RAG-2 recombinases
- DNA ligases
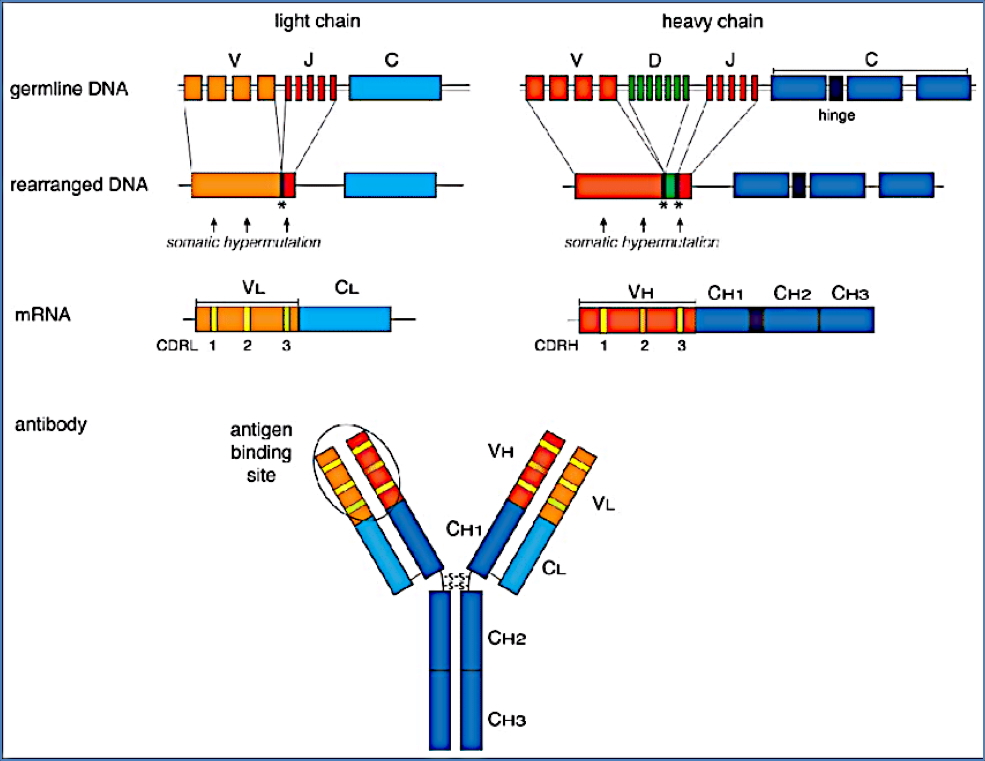
Step 2 – Surface Expression of BCRs (IgM and IgD)
- After successful gene rearrangement, B-cell expresses surface-bound IgM and IgD as B-cell receptors (BCRs)
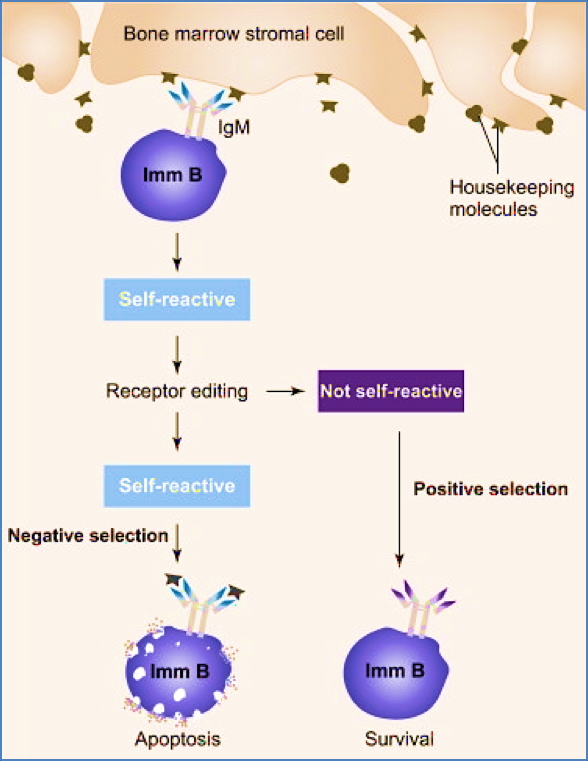
Step 3 – Negative Selection
- Immature B-cells are tested for autoreactivity
- B-cells that bind self-antigen strongly are deleted or inactivated to prevent autoimmunity
Migration to Peripheral Lymphoid Organs
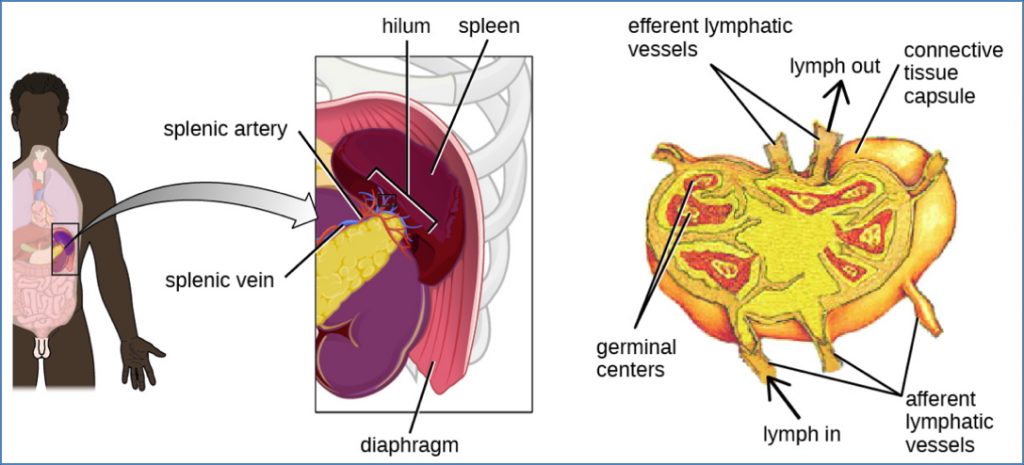
Step 4 – Homing to Secondary Lymphoid Organs
- Mature naïve B-cells leave the bone marrow and enter:
- Lymph nodes, spleen, and MALT (mucosa-associated lymphoid tissue)
- Entry into T-cell zones via high endothelial venules (HEVs)
- Guided by chemokines and adhesion molecules
- If no antigen is encountered, B-cells recirculate
Antigen Encounter and B-Cell Activation
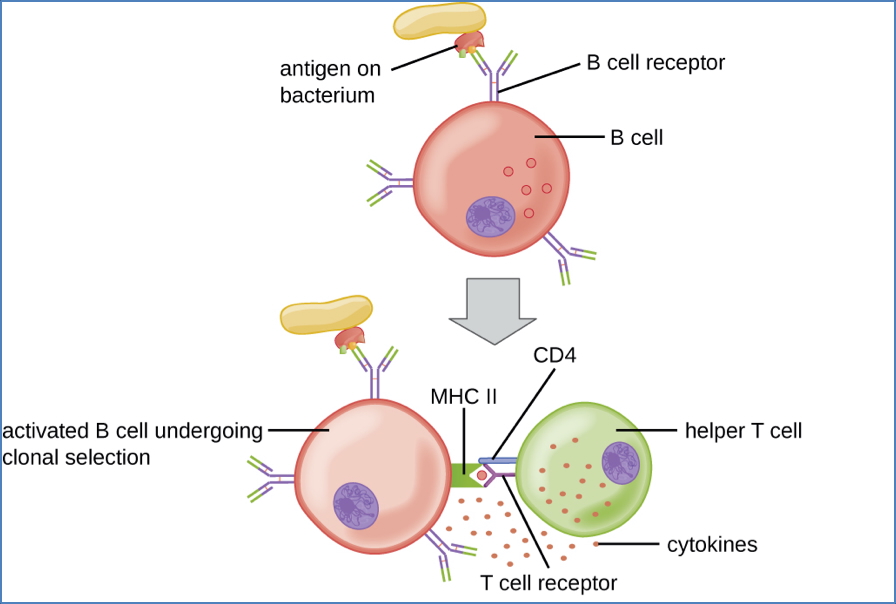
Step 5 – Activation in T-Cell Zones
- Initiated when BCRs cross-link with specific antigen
- Internalised antigen is processed and presented on MHC Class II
Two Pathways:
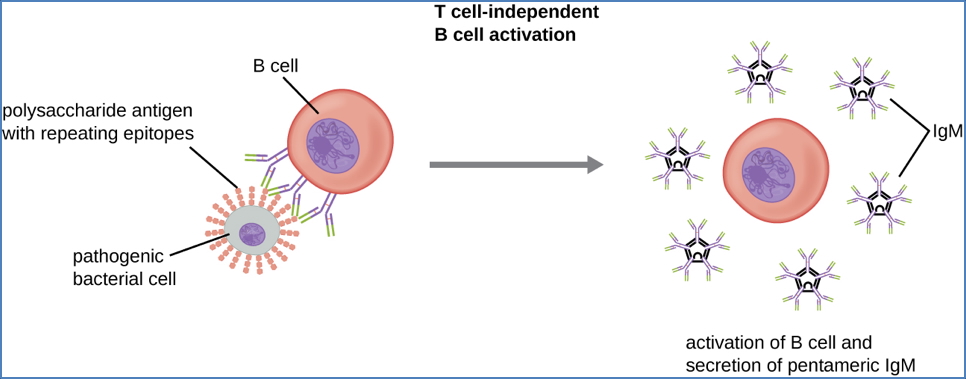
1. Thymus-Independent Activation
- B-cell activated without T-cell help
- Triggered by repetitive or polymeric antigens (e.g. bacterial polysaccharides)
2. Thymus-Dependent Activation
- Requires CD4+ T-helper cell (Th2) assistance
- 3 signals are required:
- pMHC-II : TCR interaction
- CD40 (B-cell) : CD40L (T-cell) binding
- Cytokine secretion by Th2 cell
- Leads to full B-cell activation
Germinal Centre Reaction
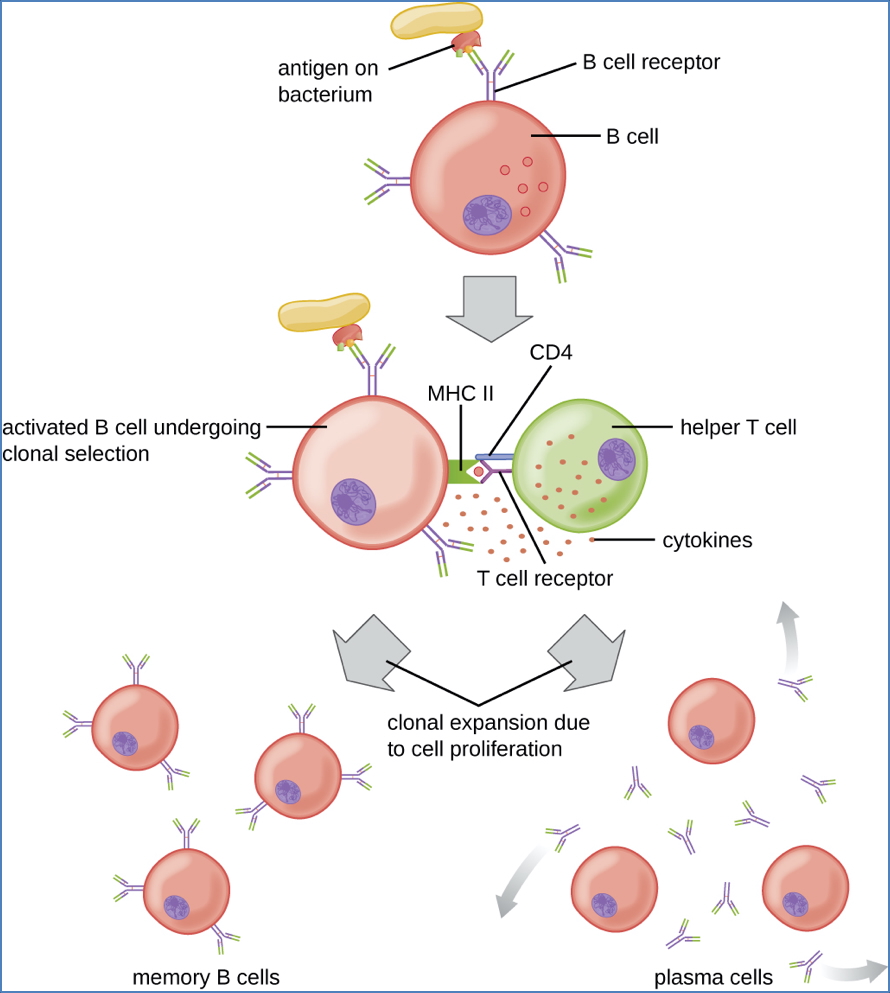
Step 6 – Differentiation and Proliferation
- Activated B-cells migrate to B-cell follicles and form germinal centres
- Undergo rapid proliferation and differentiation into:
- Plasma cells (antibody-secreting)
- Memory B-cells
Secondary Antibody Diversification Mechanisms:
Somatic Hypermutation (Affinity Maturation)
- Occurs in variable (V) regions of Ig genes
- Introduces single amino acid mutations
- Outcomes:
- ↑ Affinity → survival and proliferation
- ↓ Affinity → apoptosis
- Ensures selection of high-affinity BCRs
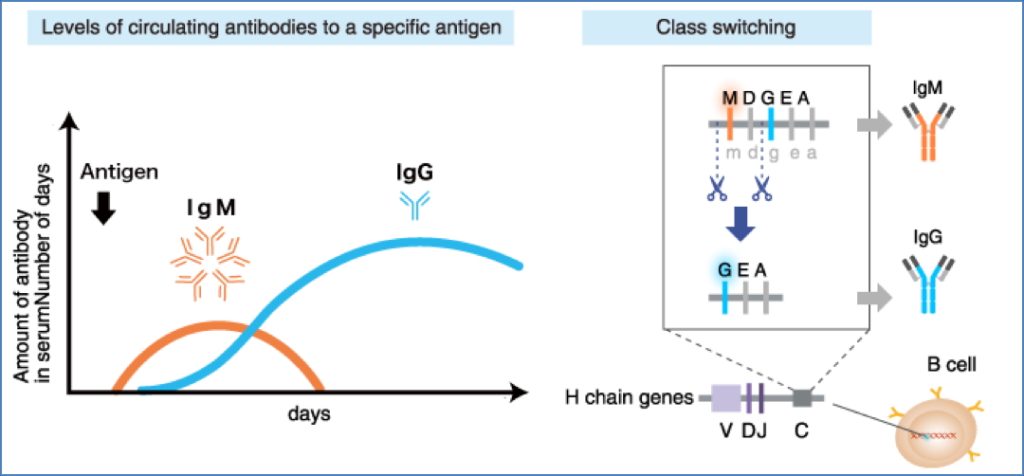
Isotype (Class) Switching
- Involves replacing the IgM constant (Fc) region with that of IgG, IgA, or IgE
- Controlled by cytokines from Th1/Th2 cells
- Requires CD40 : CD40L interaction
- Alters effector function, not antigen specificity
- See Antibodies for further detail
Final Maturation
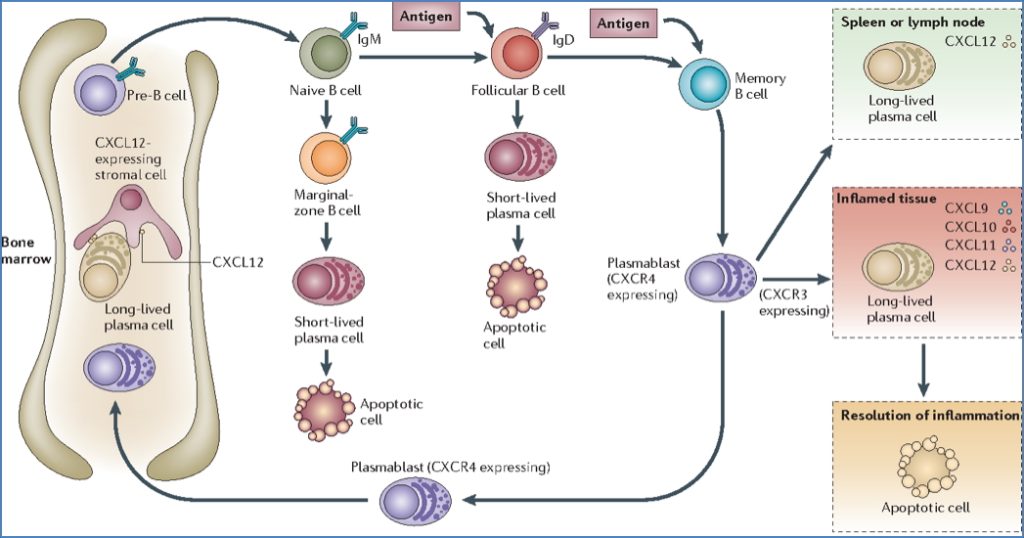
Step 7 – Plasma Cell Formation
- Long-lived plasma cells migrate to bone marrow or medullary cords
- Function: continuous antibody secretion
- Some B-cells differentiate into memory B-cells for rapid future responses
Summary – B-Cell Development
B-cell development begins in the bone marrow with immunoglobulin gene rearrangement and negative selection. Mature naïve B-cells then migrate to secondary lymphoid tissues, where antigen exposure triggers activation, clonal expansion, affinity maturation, class switching, and differentiation into plasma or memory cells. These steps are critical to mounting an effective humoral immune response. For more context, visit our Immune & Rheumatology Overview page.