Table of Contents
Overview – Glucocorticoids
Glucocorticoids are synthetic or natural corticosteroids with potent anti-inflammatory and immunosuppressive properties. These drugs act by modulating gene transcription and cytokine expression, making them central to the management of chronic inflammatory, autoimmune, allergic, and endocrine disorders. Common clinical uses include asthma, rheumatoid arthritis, Addison’s disease, and prevention of transplant rejection.
Definition
Glucocorticoids are a class of adrenal steroids that act via glucocorticoid receptors (GRα) to suppress immune responses and inflammation. While they share some activity with mineralocorticoid receptors, synthetic glucocorticoids are designed for selectivity and therapeutic efficiency.
Classical Agents
Natural
- Cortisol (Hydrocortisone) – rapidly inactivated by renal enzymes
Synthetic (Enhanced potency, half-life, and oral absorption)
- Fluticasone (common in inhalers for asthma)
- Budesonide
- Mometasone
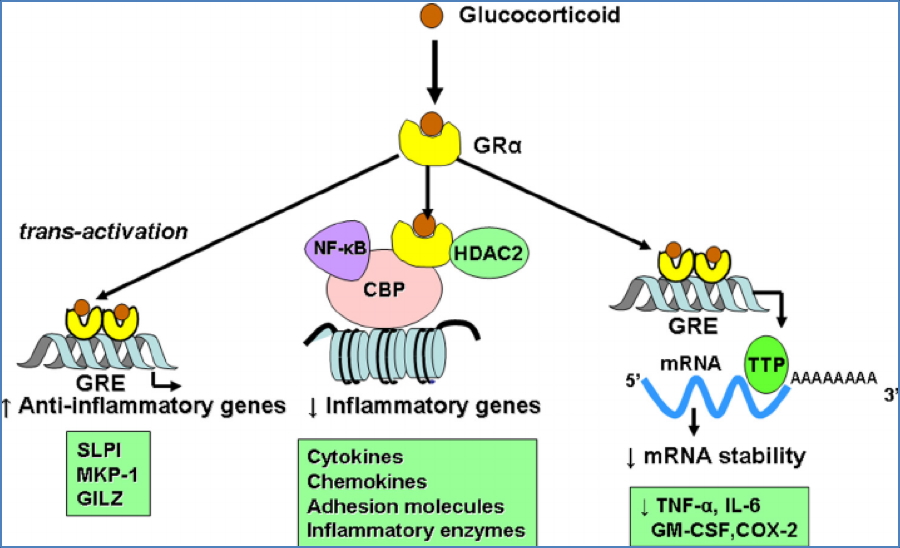
Mechanism of Action
- Activate GRα receptors → translocate to nucleus → alter gene transcription
- ↓ Cytokine expression → ↓ inflammation
- Inhibit COX-2 → ↓ prostaglandin synthesis → ↓ vasodilation and immune cell migration
- ↑ β2-receptor expression → ↑ adrenergic responsiveness
- ↓ IL-3 → ↓ mast cell proliferation → ↓ hypersensitivity reactions
Clinical Uses
- Chronic Inflammatory & Autoimmune Diseases
- Asthma (inhaled corticosteroids like fluticasone)
- Rheumatoid arthritis
- Inflammatory bowel disease
- Systemic lupus erythematosus
- Post-organ transplant immunosuppression
- Endocrine Conditions
- Addison’s disease (primary adrenal insufficiency)
Routes of Administration
- Oral
- Inhaled (topical pulmonary)
- Topical (dermatologic)
- Intra-articular
- Parenteral (IM or IV)
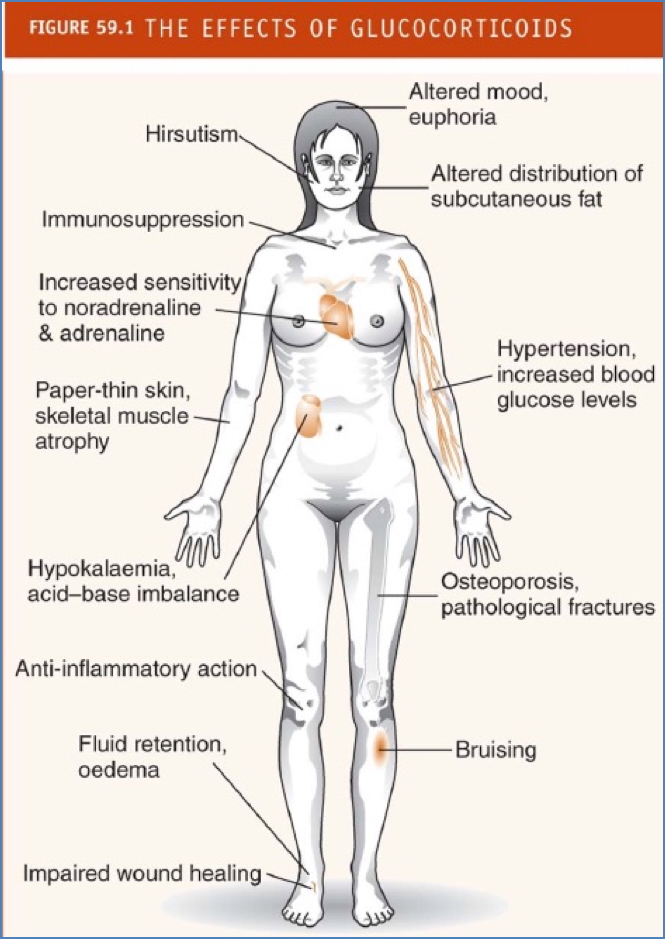
Systemic Side Effects
- Immunosuppression → ↑ infection & cancer risk
- Hypertension (via ↑ sympathetic sensitivity and fluid retention)
- Fluid retention, oedema
- Cushingoid appearance:
- Central obesity
- “Moon” face
- “Buffalo hump”
- Osteoporosis → pathological fractures
- Skin thinning, bruising, poor wound healing
- Hirsutism
- Muscle atrophy

Corticosteroids & Rheumatoid Arthritis
Pathophysiology
- Autoimmune deposition of rheumatoid factor (IgM against IgG) → chronic joint inflammation
- Cytokines (TNFα, IL-1, IL-6) drive joint destruction via osteoclasts and immune cell infiltration
Treatment
- Corticosteroids: ↓ cytokine production and immune activation
- NSAIDs: symptom relief
- DMARDs (e.g. methotrexate, leflunomide, cyclosporin): inhibit immune cell proliferation
- Biologic agents: inhibit TNFα, IL-1, IL-6, or T-cell co-stimulation
Corticosteroids & Asthma
Pathophysiology
- Type I hypersensitivity → IgE-mediated mast cell degranulation → histamine, leukotrienes, prostaglandins
- Leads to bronchoconstriction and submucosal inflammation
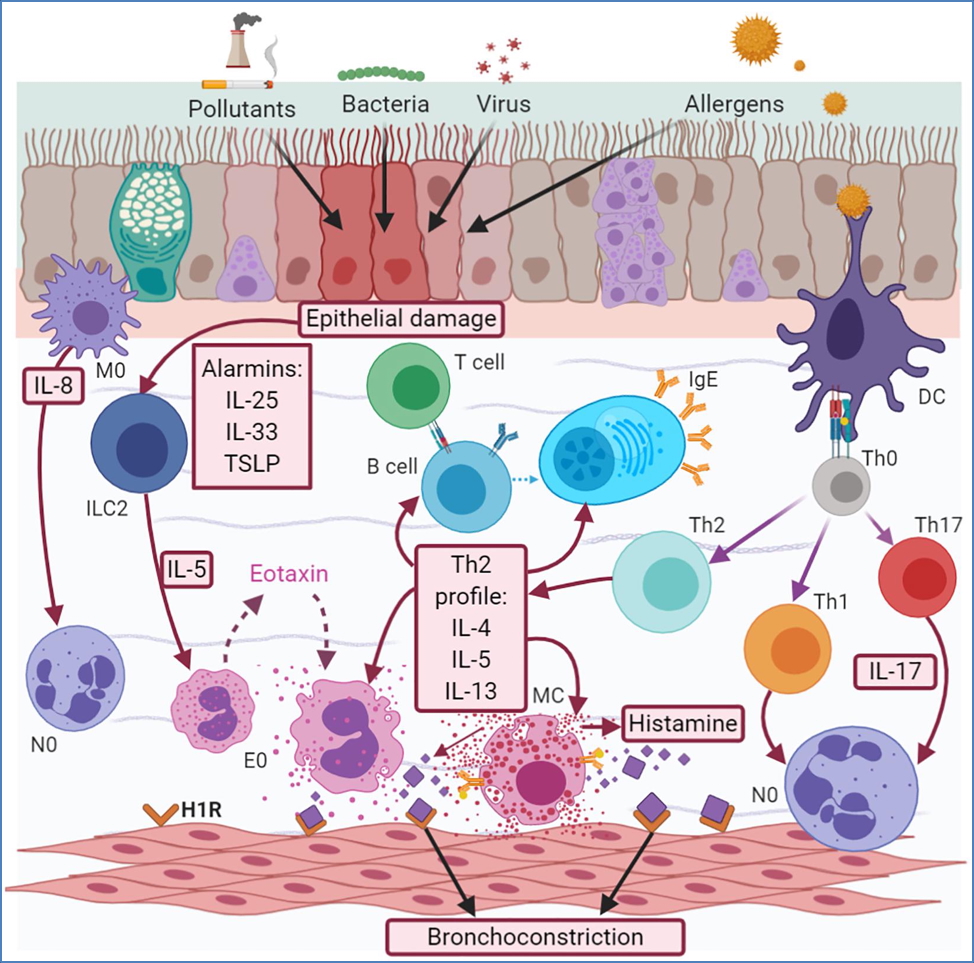

Treatment
- Inhaled corticosteroids (e.g. fluticasone, budesonide):
- ↓ cytokines and inflammatory mediators
- ↑ β2-receptor sensitivity
- ↓ mast cell proliferation

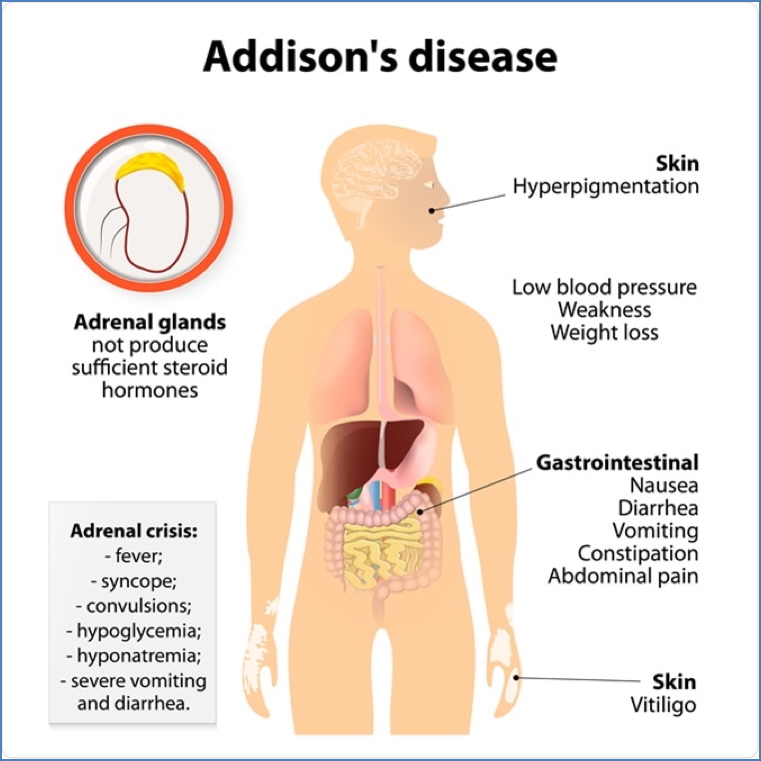
Corticosteroids & Addison’s Disease
Pathophysiology
- Adrenal insufficiency (autoimmune or TB-related) → ↓ endogenous cortisol
Symptoms
- Weakness, hypoglycaemia, hypotension, weight loss, hyperpigmentation, electrolyte imbalance
Treatment
- Hydrocortisone 15 mg AM + 5 mg PM
- Fludrocortisone 0.05–0.1 mg/day (mineralocorticoid replacement)

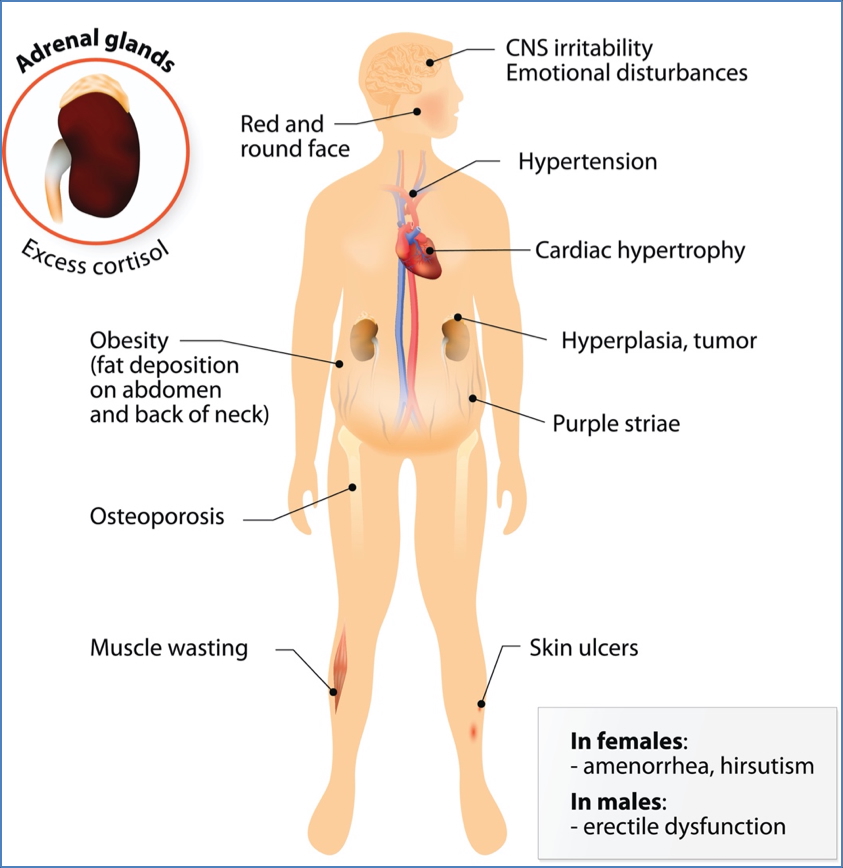
Corticosteroids & Cushing’s Syndrome
Pathophysiology
- Chronic cortisol excess (endogenous or exogenous)
- ACTH-secreting pituitary adenoma, adrenal tumours, or prolonged glucocorticoid therapy
Symptoms
- Central obesity
- Moon face
- Buffalo hump
- Hypertension
- Fragile skin, bruising
- Osteoporosis
Treatment
- Gradual withdrawal if iatrogenic
- Surgery + temporary steroid replacement if tumour-related

Summary – Glucocorticoids
Glucocorticoids are potent anti-inflammatory and immunosuppressive agents used across multiple clinical domains including asthma, rheumatoid arthritis, and Addison’s disease. Their mechanism hinges on transcriptional regulation via GRα receptors, but prolonged use carries significant systemic side effects. For a broader context, see our Pharmacology & Toxicology Overview page.