Table of Contents
Overview – Cell Injury and Death
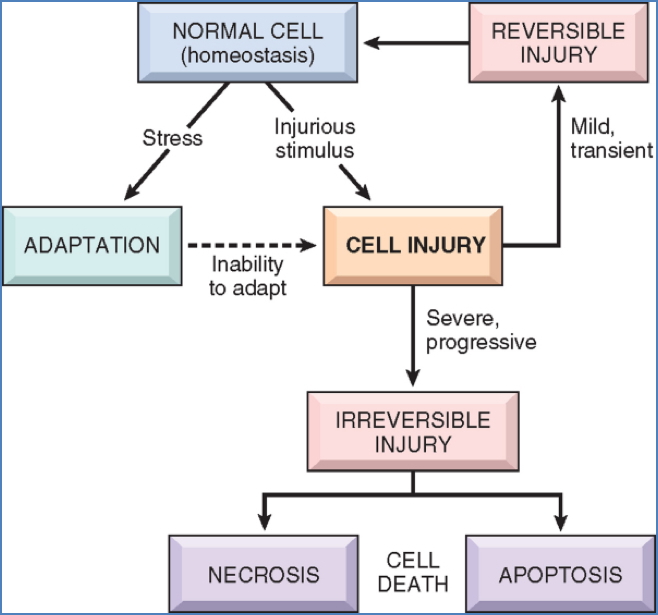
Cell injury and death occur when cells are exposed to noxious stimuli beyond their capacity to adapt. Initially, this damage may be reversible, but persistent or severe stress can lead to irreversible injury and cell death. Understanding the mechanisms of injury — particularly ischaemic, toxic, and oxidative insults — is crucial to recognising the pathogenesis of many clinical conditions, from infarction to inflammation and organ failure.


Definition
Cell injury is the functional and structural damage sustained by a cell when its adaptive responses are overwhelmed.
- Reversible injury: Recovery is possible if the stimulus is removed early.
- Irreversible injury: Leads to cell death via necrosis or apoptosis.
Causes of Cell Injury
- Oxygen Deprivation (Hypoxia/Ischaemia)
- Most common clinical cause
- ↓ Aerobic respiration → ↓ ATP
- Causes: Ischaemia (↓ blood flow), hypoxaemia, anaemia, CO poisoning
- Physical Agents
- Trauma, temperature extremes, radiation, pressure, electric shock
- Chemical Agents & Drugs
- Acids, bases, poisons (e.g. cyanide, mercury)
- Infectious Agents
- Viruses, bacteria, fungi, parasites
- Immunological Reactions
- Autoimmune diseases, hypersensitivity reactions
- Genetic Derangements
- Mutations affecting essential proteins
- Nutritional Imbalances
- Vitamin deficiencies or excesses, obesity, malnutrition

Centro-Lobular Hepatocyte Vulnerability
Centro-lobular hepatocytes are more susceptible to injury due to:
- Receiving low-oxygen, nutrient-poor, waste-laden blood from peripheral zones
- Blood pooling in this region (slow drainage via central vein) → Prolonged toxin exposure
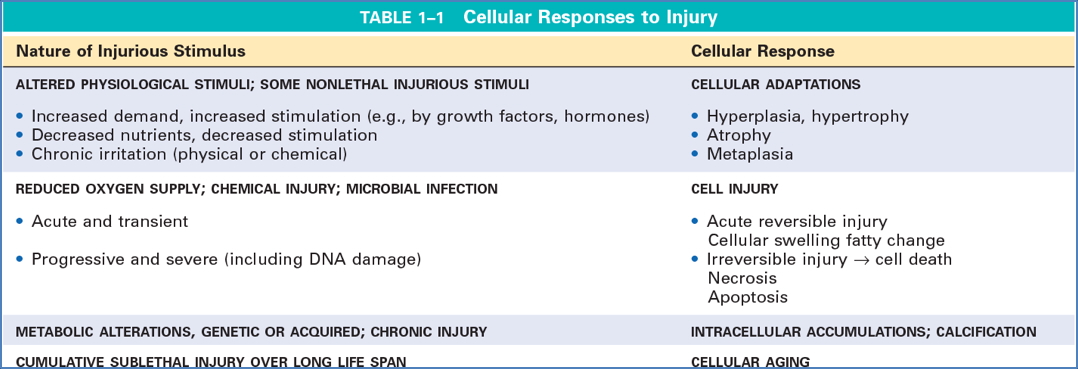
Biochemical Mechanisms of Cell Injury
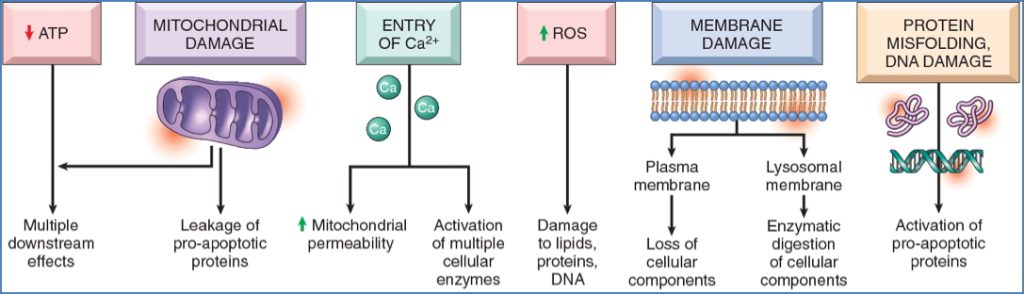
1. ATP Depletion
- ↓ ATP impairs:
- Na+/K+ pump → cell swelling
- Ca²⁺ pump → ↑ cytosolic Ca²⁺
- Protein synthesis, lipogenesis
- Anaerobic metabolism → lactic acid build-up → ↓ pH
- Ultimately → irreversible mitochondrial/lysosomal damage


2. Mitochondrial Damage
- Caused by:
- Hypoxia, toxins, free radicals
- Consequences:
- Formation of permeability transition pores → collapse of membrane potential
- Cytochrome c release → caspase activation → apoptosis
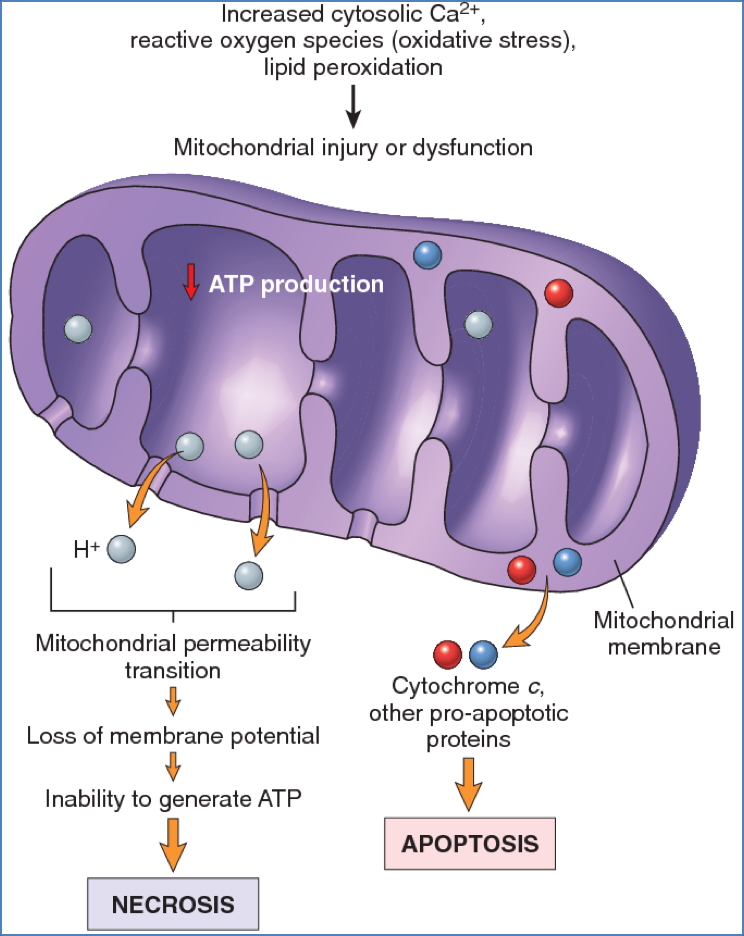

3. Loss of Calcium Homeostasis
- ↑ Intracellular Ca²⁺ due to:
- ATP-dependent pump failure
- Release from mitochondria/ER
- Effects:
- Activation of enzymes (ATPases, phospholipases, proteases, endonucleases)
- Induction of mitochondrial permeability
- Caspase activation → apoptosis


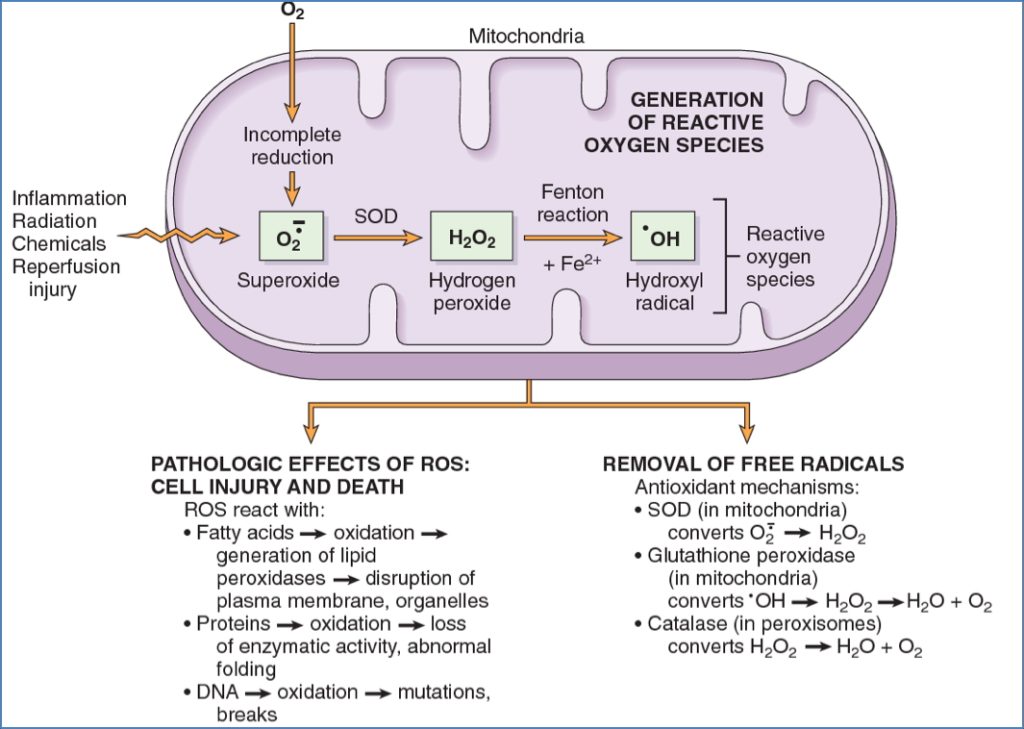
4. Oxidative Stress (Free Radicals / ROS)
- Sources:
- Mitochondrial respiration, inflammation, radiation, toxins, reperfusion
- Consequences:
- Lipid peroxidation of membranes
- Oxidative modification of proteins
- DNA strand breaks and mutations
- Cell death by necrosis or apoptosis

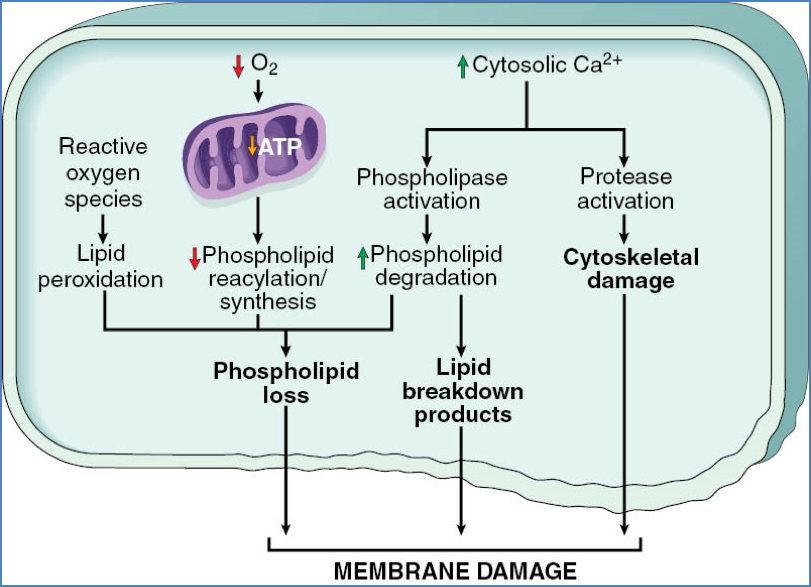
5. Membrane Damage
- Caused by:
- ATP depletion → ↓ phospholipid synthesis
- Ca²⁺ activation of phospholipases
- Free radicals, toxins, complement, perforins
- Consequences:
- Mitochondrial → cytochrome c release
- Plasma → ion/fluid influx, protein leakage
- Lysosomal → release of RNAses, DNAses, proteases → necrosis

Ischaemic and Hypoxic Injury
Ischaemia
- ↓ Blood flow → ↓ O₂ and nutrients
- Anaerobic glycolysis eventually stops (no substrates)
Hypoxia
- ↓ O₂ only (e.g. respiratory failure)
- Anaerobic glycolysis may continue
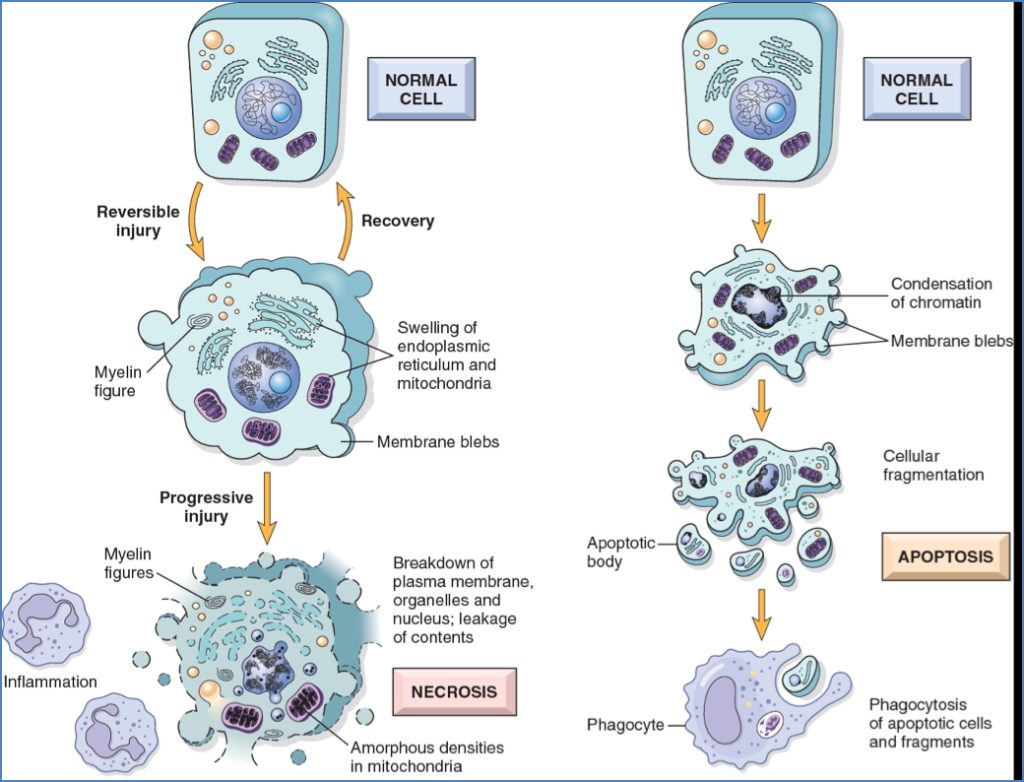
Reversible Injury
- ↓ ATP, Na⁺/K⁺ pump failure, cell swelling
- Lactic acidosis, ↓ pH
- ↓ Protein synthesis
- Ultrastructural changes: blebs, chromatin clumping
Irreversible Injury
- Massive Ca²⁺ influx
- Mitochondrial swelling, lysosomal rupture
- Membrane rupture → enzyme leakage (e.g. troponin, CK)
- Myelin figures → phagocytosis or calcification
Ischaemia-Reperfusion Injury
- Injury worsens after reflow due to:
- ROS generation
- Complement activation


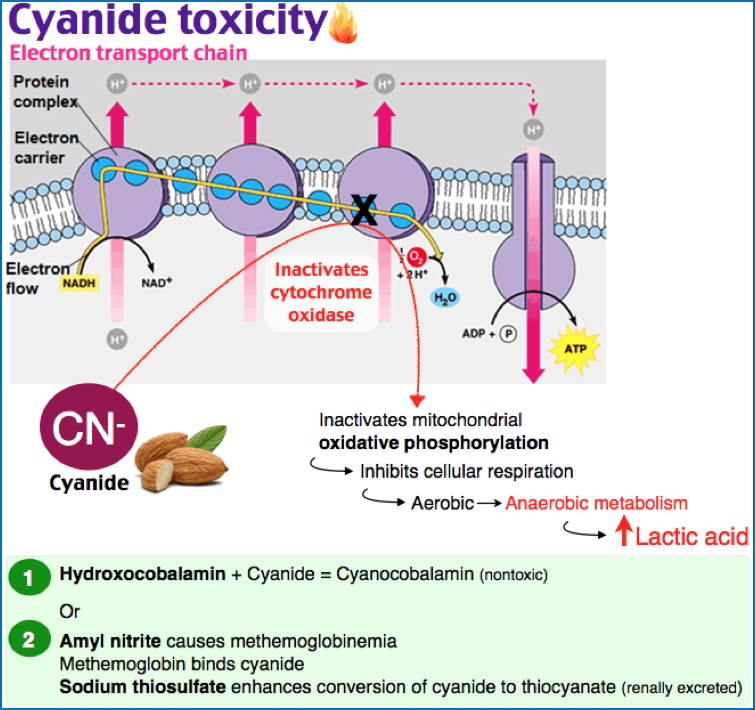
Chemical Injury
- Direct damage:
- Mercury → binds membrane proteins → ↑ permeability
- Cyanide → inhibits cytochrome oxidase → halts ATP synthesis

Morphological Changes in Cell Injury
Reversible
- Cellular swelling (hydropic change)
- Na⁺/K⁺ pump failure
- Fatty change
- In liver/myocardium from disrupted lipid metabolism
- Other features:
- Blebbing, ribosome detachment, nuclear chromatin clumping
Irreversible – Necrosis
- Loss of membrane integrity → leakage → inflammation
Nuclear Changes
- Pyknosis: nuclear shrinkage
- Karyorrhexis: fragmentation
- Karyolysis: fading due to DNA degradation


Patterns of Necrosis
Coagulative Necrosis
- Most common
- Preserved cell outlines, firm tissue
- Seen in infarcts (except brain)
- E.g. MI, gangrene
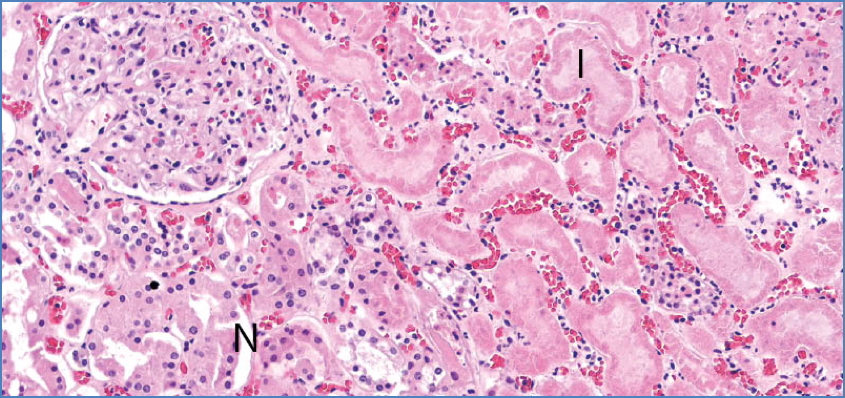

Calicut Medical College, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Liquefactive Necrosis
- Enzymatic digestion dominates
- Pus formation, abscess
- Also seen in CNS infarcts
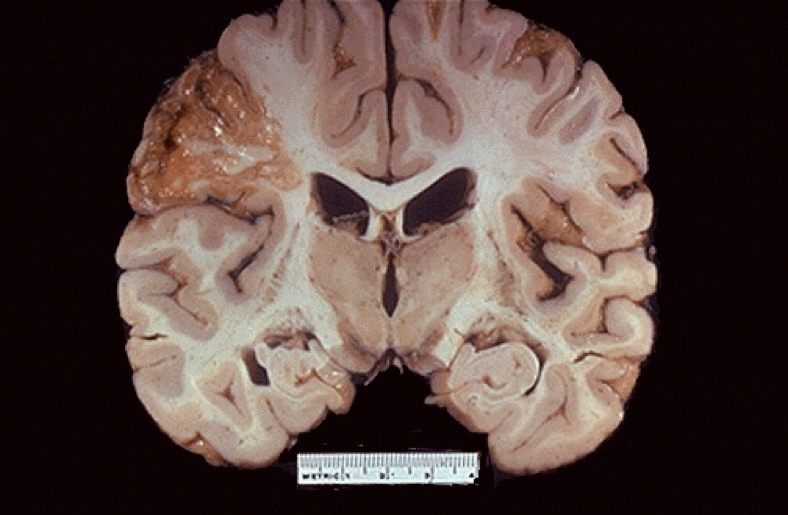

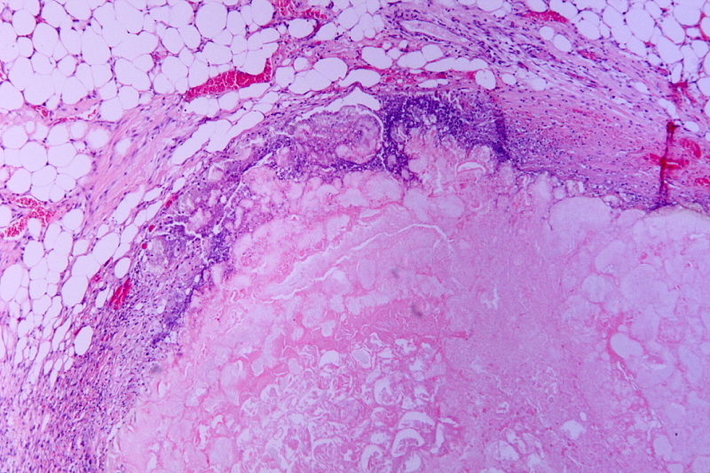
Caseous Necrosis
- Granulomatous, cheese-like
- Seen in TB, fungi
- Surrounded by inflammatory border
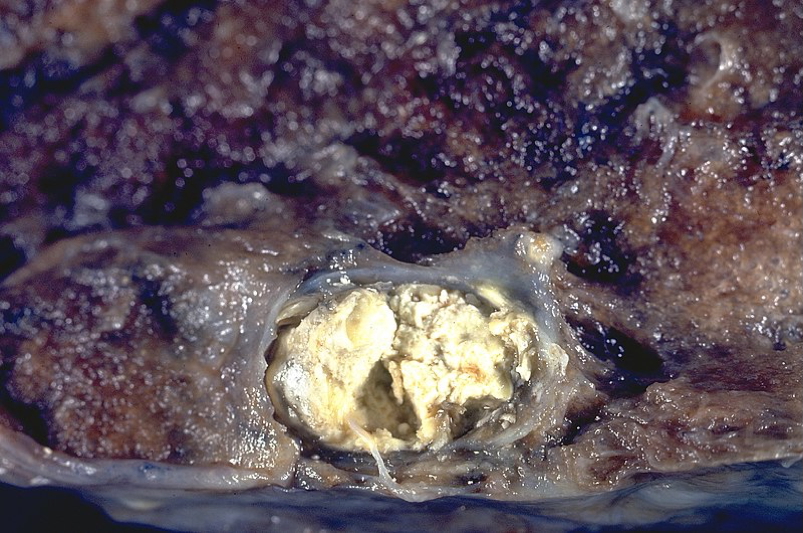

Fat Necrosis
- Lipase release → fat breakdown
- E.g. acute pancreatitis, breast trauma
- Chalky-white deposits

Apoptosis
- Regulated cell death without inflammation
- Fragmentation into apoptotic bodies
- Physiological:
- Embryogenesis, immune cell culling, menstruation
- Pathological:
- DNA damage, misfolded proteins, viral infections

Summary – Cell Injury and Death
Cell injury and death result from a range of noxious stimuli including hypoxia, toxins, and oxidative stress. These processes involve key molecular events such as ATP depletion, mitochondrial dysfunction, calcium overload, and ROS generation. The outcome can be reversible injury, necrosis, or apoptosis. For foundational topics, see our Cell Biology & Biochemistry Overview page.