Table of Contents
Overview
Selective toxicity is the foundational principle behind antimicrobial therapy—it refers to the ability of a drug to target harmful microorganisms without damaging host cells. This concept enables the safe treatment of infections by exploiting structural and functional differences between pathogens and human cells. Understanding how selective toxicity works is crucial for prescribing effective antimicrobials while minimising side effects and resistance.
Definition
Selective toxicity is the ability of a drug to harm a pathogen without harming the host. It is essential for the effectiveness and safety of antimicrobial agents.
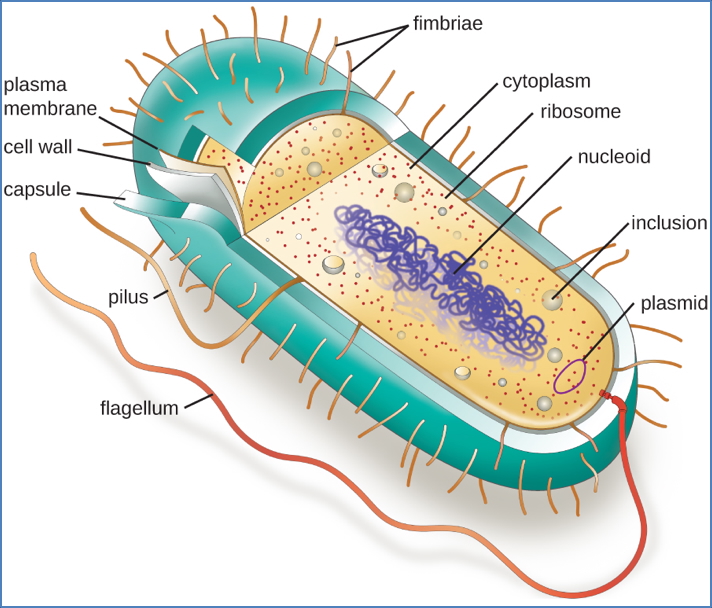
Microbial Cell Biology & Implications
Host Cells
- Human cells = Eukaryotes
- Membrane-bound nucleus and organelles


Bacteria
- Prokaryotes
- Lack nucleus and organelles → Highly different from human cells
- Enables strong selective toxicity for antibacterials
- Gram-positive bacteria:
- Thick peptidoglycan cell wall
- Gram-negative bacteria:
- Thin peptidoglycan, outer lipopolysaccharide (LPS) layer
- Harder to penetrate with antibiotics

Fungi & Parasites
- Eukaryotes
- Similar to human cells → Harder to target without host toxicity
- Explains higher side effect profiles of antifungals and antiparasitics
Viruses
- Obligate intracellular pathogens
- Rely on host-cell machinery for replication
- Antivirals often affect host processes → Limited selective toxicity


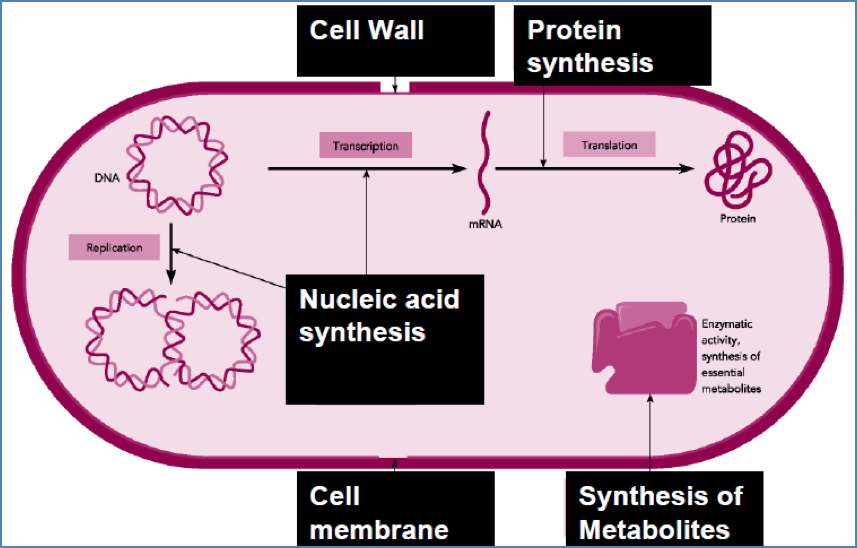
Principle of Antimicrobial Therapy
Origins
- Many antimicrobials are derived from natural organisms
- Penicillin discovered from Penicillium mold
Selective Toxicity in Action
- Targets features unique to pathogens (e.g. bacterial cell walls, viral enzymes)
- Reduces risk to host tissue
Spectrum of Activity
- Bactericidal: Kills bacteria
- E.g. Penicillin
- Bacteriostatic: Inhibits bacterial growth
- E.g. Tetracycline
- Preferred in sepsis, to avoid endotoxin release
- Synergistic combinations:
- E.g. Aminoglycosides + β-lactams
- β-lactam damages wall → enhances aminoglycoside entry
- E.g. Aminoglycosides + β-lactams
Empirical vs Directed Therapy
Broad-Spectrum (Empirical)
- Covers Gram-positive + Gram-negative
- Used when pathogen is unknown
Narrow-Spectrum (Directed)
- Targets specific microbes
- Requires organism identification
- Benefits:
- More effective
- Less impact on normal flora
- Reduces antibiotic resistance
Antimicrobial Resistance
- Bacteria evolve rapidly → High mutation rate
- Can share resistance genes (via plasmids, conjugation)
- Selective pressure from antibiotics → Promotes survival of resistant strains
“Restraint in antimicrobial use is the best way to ensure long-term efficacy.”
Common Sites of Selective Toxicity

Summary
Selective toxicity underpins antimicrobial therapy by allowing drugs to specifically target microbial structures without damaging host tissues. Understanding microbial differences helps tailor effective, evidence-based treatments while minimising side effects and resistance. For a broader context, see our Pharmacology & Toxicology Overview page.