Table of Contents
Overview – Future Genetic Therapies
Future genetic therapies are revolutionising how we understand, prevent, and treat disease at the molecular level. By leveraging genomic analysis, personalised gene-editing, and targeted delivery systems, these technologies are shifting medicine from reactive to predictive and regenerative. From reverse vaccinology to human artificial chromosomes and gene therapy vectors, final-year medical students must understand these cutting-edge approaches as they prepare for careers in a rapidly evolving healthcare landscape.
Understanding Pathogen Genomics
Beyond Traditional Immunisation
- Historically, vaccines and vector control were the main strategies against infectious diseases.
- In the future, genome analysis of pathogens and hosts will enable:
- Development of targeted insecticides (e.g. disabling vector transmission).
- Creation of drugs targeting specific metabolic enzymes in pathogens.
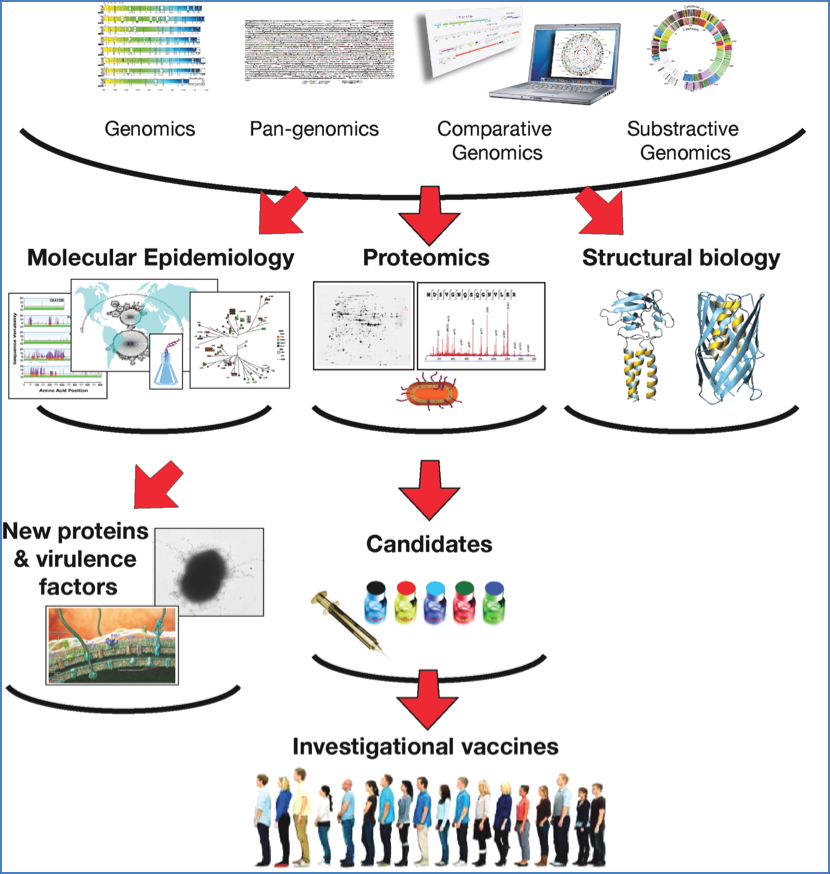
- Vaccine design via reverse vaccinology:
- Compare pathogen genomes → identify potential antigens.
- Use proteomics, epidemiology, and structural biology to select vaccine candidates.

Organ and Tissue Engineering
Stem Cell Therapies
- Tissues engineered from a patient’s own cells → no immune rejection.
- Stem cells can be sourced from embryos, cord blood, or adult tissues.
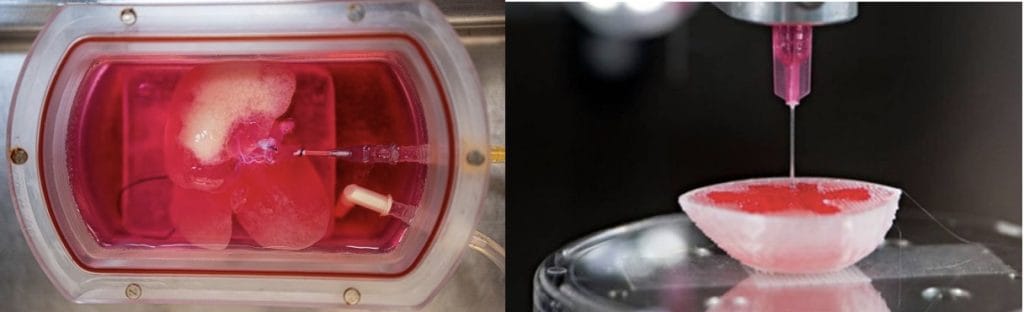
Tissue Engineering Strategies
- Biomaterial scaffolds: Provide structure and promote cell organisation.
- Cells alone: Capable of forming tissues without scaffolds.
- Cells on scaffolds: Mimic physiological environments for organ development.
- Goal: Custom-made organs → potential end to organ donation dependency.

Gene Therapy Techniques
Somatic Gene Therapy
- Insertion of a normal gene into cells to produce a missing or dysfunctional protein.
- Best suited for recessive disorders (e.g. haemophilia, cystic fibrosis).
- Dominant disorders are harder to treat due to need to remove harmful alleles.
Germline Gene Therapy
- Genetic modifications to germ cells (e.g. sperm, eggs).
- Ethically controversial and not currently approved in humans.


Methods of Gene Delivery
Mechanical & Chemical
- Liposomes: Vesicles containing therapeutic DNA fuse with cells and release contents.
- Microprojectiles: DNA-coated metal particles are physically shot into tissues.


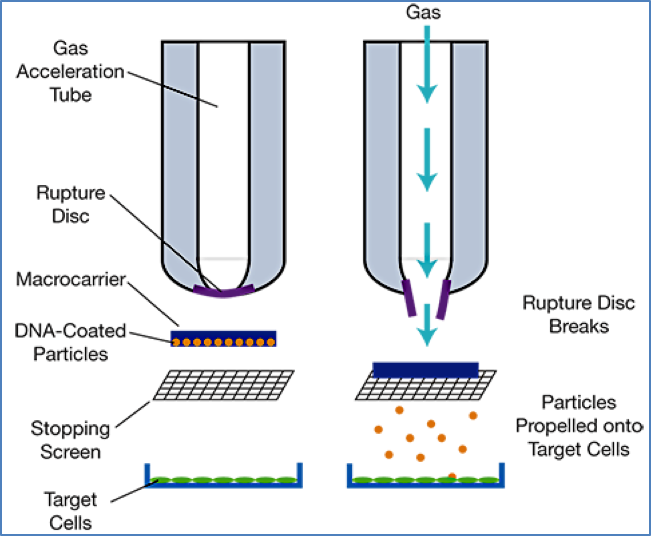

2. https://www.aiche.org/resources/publications/cep/2017/april/delivering-genes-plants
RNA-Based Therapy
- Small interfering RNA (siRNA):
- 21–23 base pair molecules.
- Bind to complementary mutated mRNA and cleave it.
- Useful for dominant traits or gain-of-function mutations.


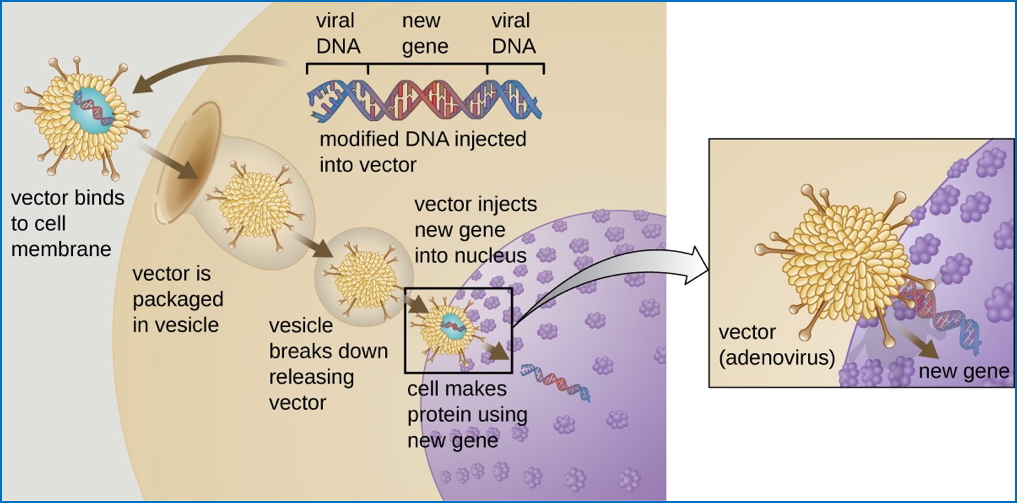
Viral Vectors
- Genes are inserted into a viral genome.
- The virus infects host cells → delivers genetic material into the nucleus.
- Expression of desired protein occurs within the host.

Vector Administration Methods
- Ex vivo: Cells are modified in the lab and reinserted.
- In situ: Vector is applied directly to affected tissue.
- In vivo: Vector administered systemically and targets specific cells.


Limitations of Viral Vectors
- Immune response from repeat exposure.
- Risk of insertional oncogenesis if virus integrates into tumour-suppressor genes.
- Containment of viral replication must be ensured.
Human Artificial Chromosomes
- Synthetic chromosomes designed to carry functional gene clusters.
- Passed on stably during cell division.
- Promising off-the-shelf solution for gene therapy.
- Current challenge: efficient delivery into human cells.


Genetic Screening and Prevention
Importance of Knowing Your Genome
- All diseases have some genetic component.
- Individualised treatment may depend on genetic predisposition.
- In future: routine genomic profiling to guide healthcare.
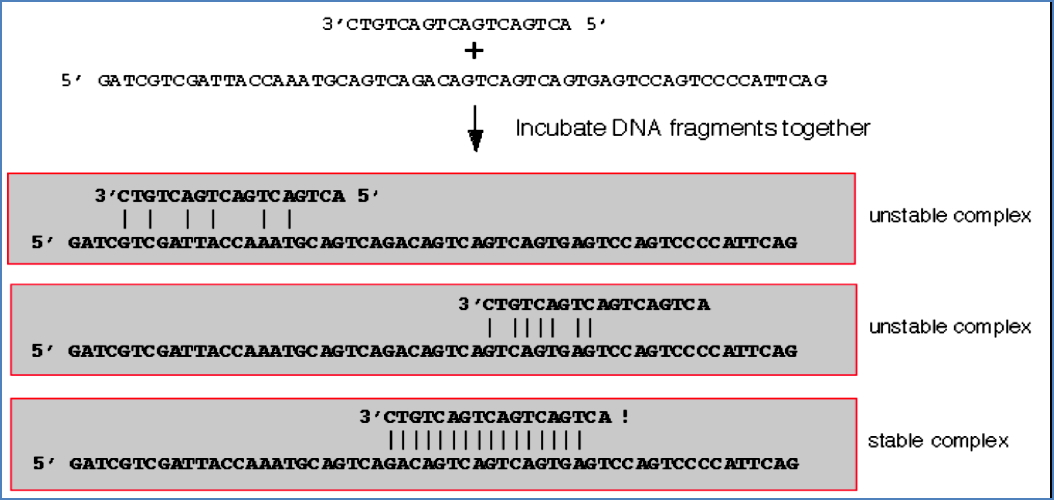
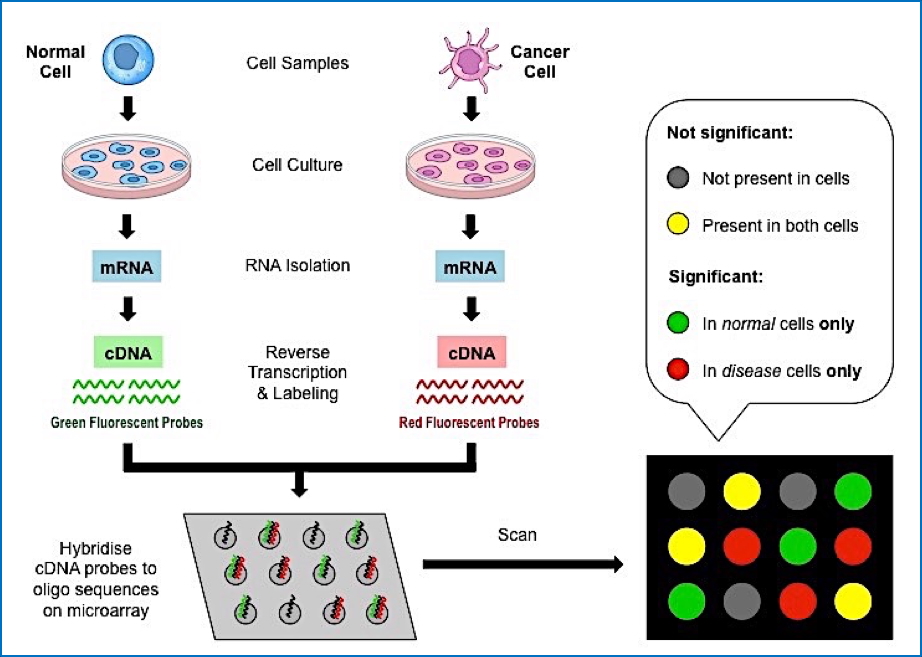
DNA Microarray Technology
- A slide embedded with thousands of immobilised DNA sequences.
- Patient’s DNA or cDNA is fluorescently labelled and hybridised onto the array.
- Hybridisation indicates presence or absence of specific sequences.


Applications
- Cancer Risk Screening
- Expression Profiling:
- Detects which genes are active in a specific tissue.
- Can compare normal vs cancerous expression profiles.
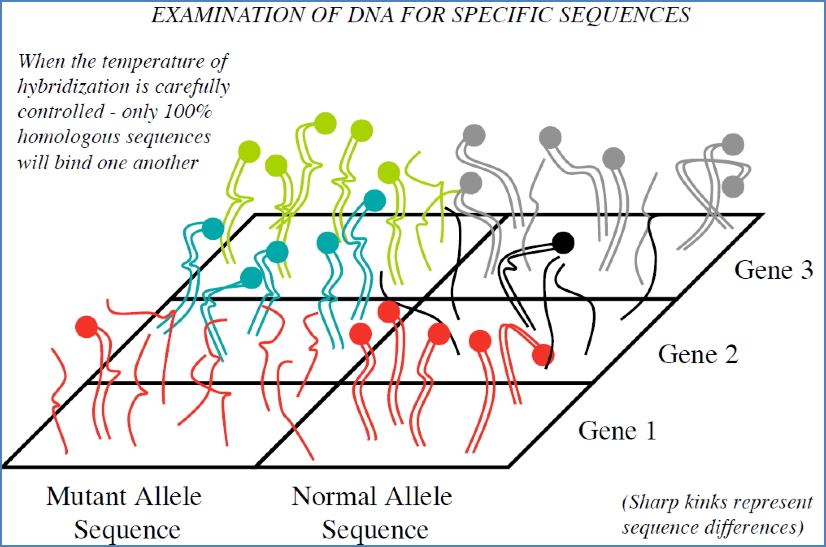
- Mutation Profiling:
- Detects disease-causing mutations.
- Homozygosity = fluorescence in one column;
- Heterozygosity = fluorescence in both normal and mutant columns.

Summary – Future Genetic Therapies
Future genetic therapies promise personalised medicine through gene editing, stem cell engineering, and advanced screening technologies. Techniques like viral vector delivery, siRNA silencing, and DNA microarrays are rapidly shifting the clinical landscape. For a broader context, see our Genetics & Cancer Overview page.